Immobilization method of candida antarctica lipase B
A technology of Candida Antarctica and lipase, applied in the field of enzyme engineering, can solve problems such as loss, and achieve the effects of convenient operation, good temperature stability and solvent tolerance, and simple preparation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Immobilization conditions of lipase B of Candida antarctica and determination method of lipase activity
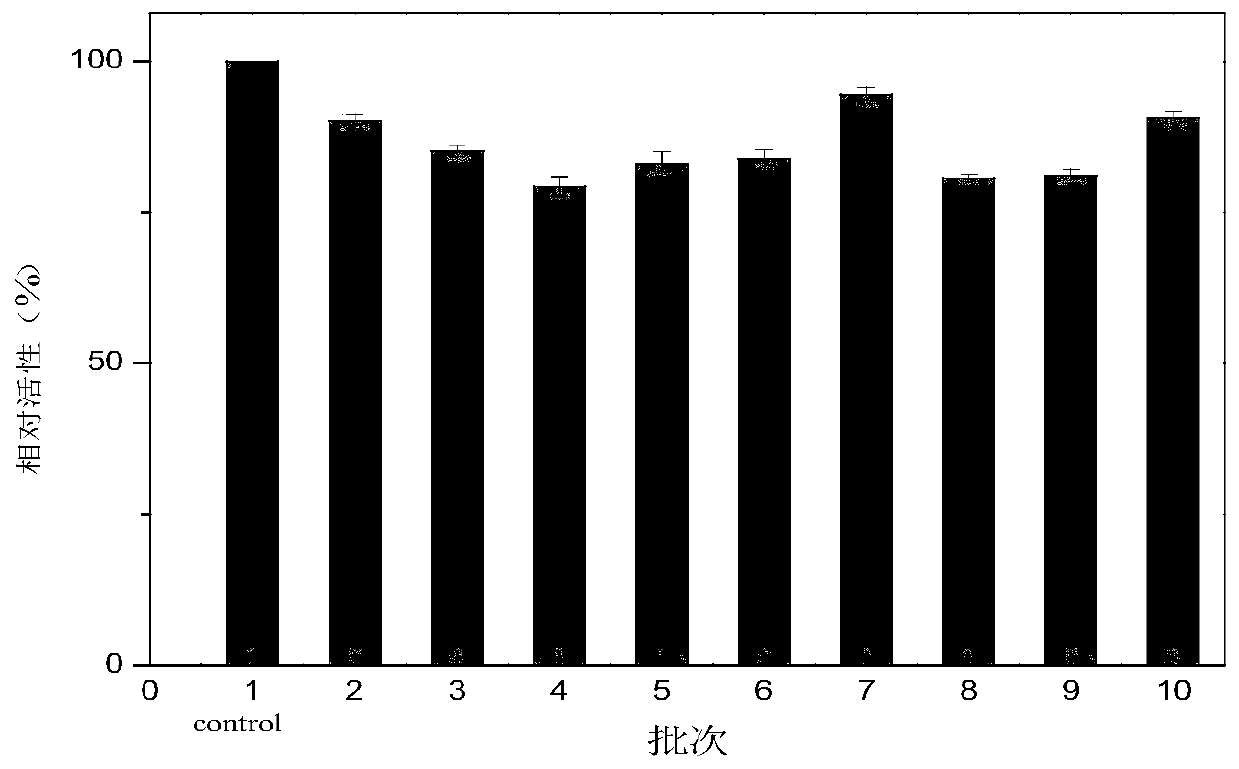
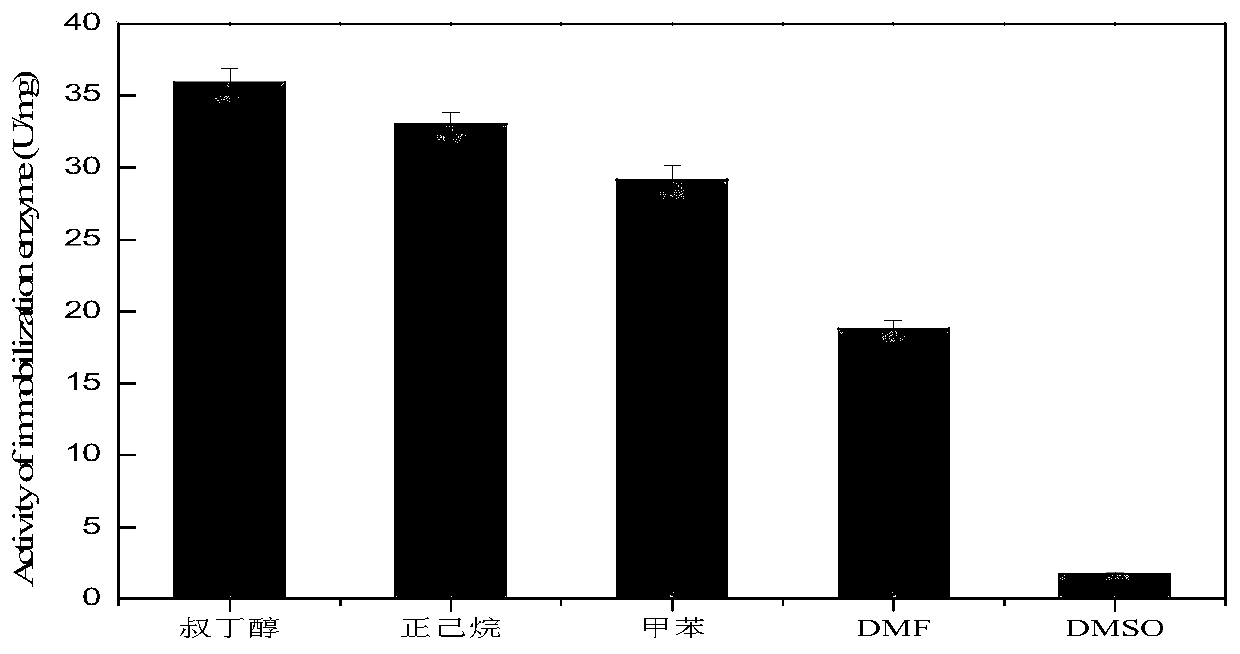
[0024] Dimethylimidazole and zinc nitrate were added into deionized water, respectively, and sonicated at 40 Hz for 20 minutes to prepare 0.3125mol / L dimethylimidazole aqueous solution and 0.3125mol / L zinc nitrate aqueous solution. Take 60mL 0.3125mol / L dimethylimidazole aqueous solution, 1.5mL 0.3125mol / L zinc nitrate aqueous solution and 3mL Candida antarctica lipase B enzyme solution (Denmark Novozymes CALB, enzyme amount 72mg / ml, 4166.67 U / ml) was mixed to obtain a mixed enzyme solution, and the reaction was magnetically stirred at 25° C. and 200 rpm for 30 min to obtain a reaction solution containing MOFs (CALB-ZIF-8) complex. Add 0.5 g of macroporous resin D101-1 and 2.5 mL of 25% polyvinylimide (PEI) aqueous solution with a mass concentration of 25% by weight to the reaction solution, cross-linking reaction for 5 hours at 30°C and 200 rpm in a water ba...
Embodiment 2
[0031] Example 2 Effect of the ratio and concentration of solution used in the preparation process of immobilized enzyme on enzyme activity
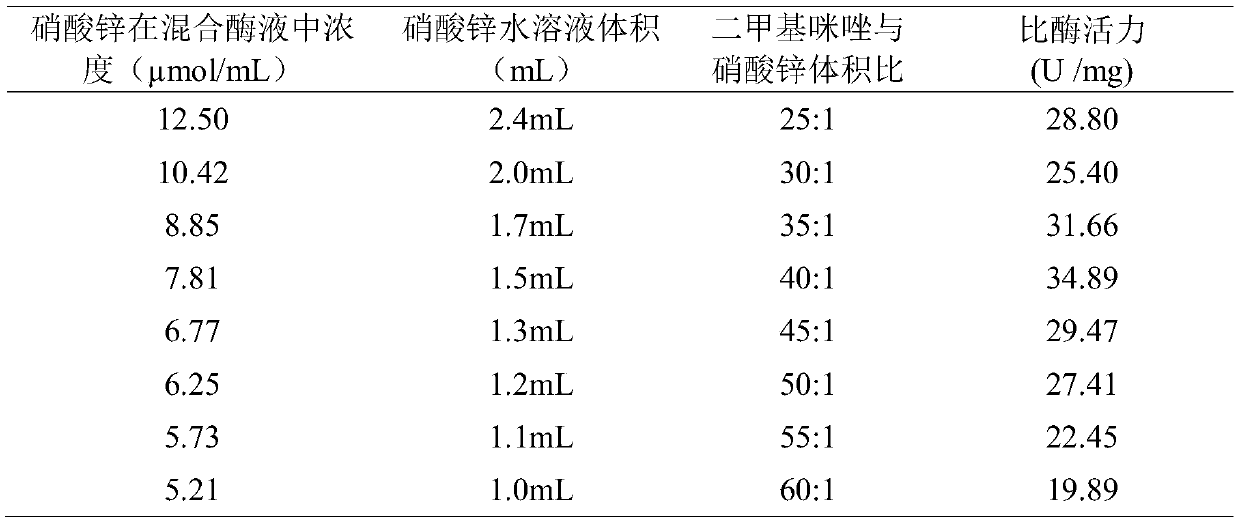
[0032] The volume of the zinc nitrate aqueous solution in Example 1 was set to 1.0 mL-2.4 mL, and the other operations and conditions were the same as in Example 1. The results are shown in Table 1. The optimal volume ratio of the dimethylimidazole aqueous solution to the zinc nitrate aqueous solution is 40: 1.
[0033] Table 1: Results of specific enzyme activity of different volumes of zinc nitrate aqueous solutions
[0034]
Embodiment 3
[0035] Example 3 Effect of the amount of enzyme solution used in the preparation process of immobilized enzyme on enzyme activity
[0036] The volume of the Candida antarctica lipase B enzyme solution in Example 1 was set to 1-6 ml, and the other operations and conditions were the same as those in Example 1. The results are shown in Table 2. The optimal amount of enzyme solution added was 3 mL (72 mg / ml).
[0037] Table 2 Results of specific enzyme activity of different volumes of enzyme solution
[0038]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com