Compound traditional Chinese medicine cataplasm for treating canine diarrhea and production process of cataplasm
A compound traditional Chinese medicine and production process technology, which is applied in the directions of drug combination, pharmaceutical formula, and medical preparations containing active ingredients, etc. Good prognosis and controllable effect of dosage and composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A kind of production of compound Chinese medicine cataplasm for treating dog diarrhea, the production technology of described compound Chinese medicine cataplasm comprises the following steps:
[0027] (1) Extraction of traditional Chinese medicine: Weigh 5g of pitted ebony, 25g of dried persimmon, 6g of myrobalan meat, 6g of coptis, 6g of turmeric, 15g of Humulus, 9g of pomegranate peel, 9g of bayberry root, and 6g of nutmeg, mix them with distilled water and boil on high heat After that, continue to decoct on low heat for 20 minutes, filter the medicinal liquid, add the medicinal residues to distilled water and boil again on high heat, then decoct on low heat for 20 minutes, filter while hot, and combine the two filtrates to obtain the compound Chinese medicinal liquid for later use;
[0028] (2) Preparation of Chinese medicinal extract: add the above-mentioned composite Chinese medicinal liquid to sodium benzoate while hot, place in an electric blast drying oven after...
Embodiment 2
[0033] According to the Chinese Pharmacopoeia Standards (2015 edition) quality standards, the appearance and shape evaluation, adhesion strength detection and weight difference evaluation of the cataplasm patch prepared in the above-mentioned Example 1 were carried out. To measure the adhesion strength, paste it on the phenolic material board with poultice, the adhesion area is 3cm×3cm, measure it with a tension meter, and record the data when the sample is pulled off the board. Stability test of poultice: place 4 samples in a constant temperature and humidity oven with a relative temperature of 37°C and a humidity of 75%, and measure the adhesion strength after taking out the samples.
[0034] The appearance evaluation results are as follows: the color of the cataplasm is dark brown, the appearance is a coin shape with a diameter of 3cm, the surface is shiny, the touch is slightly elastic and sticky, and it is applied on a square non-woven fabric with a side length of 7cm.
...
Embodiment 3
[0038] Detect the therapeutic effect and mechanism of the above-mentioned cataplasma, the detection method is as follows:
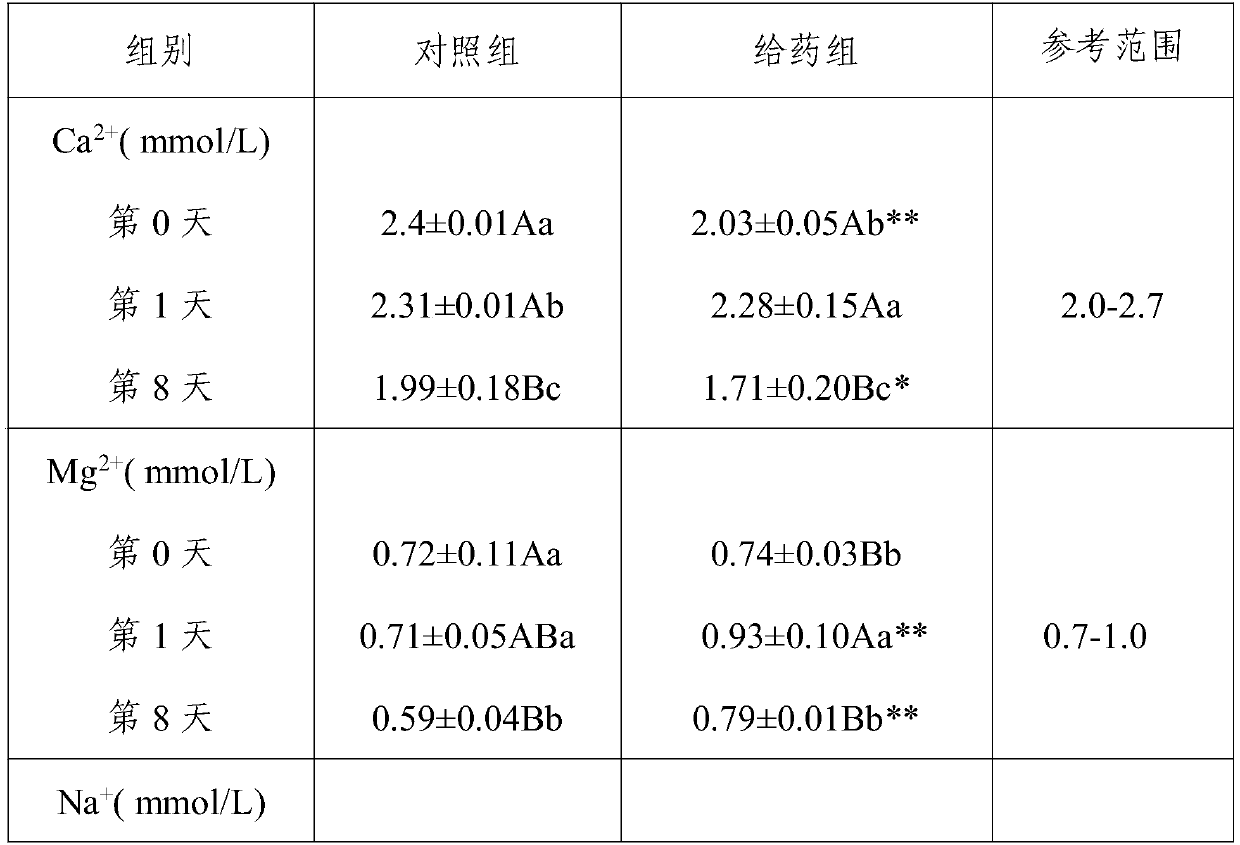
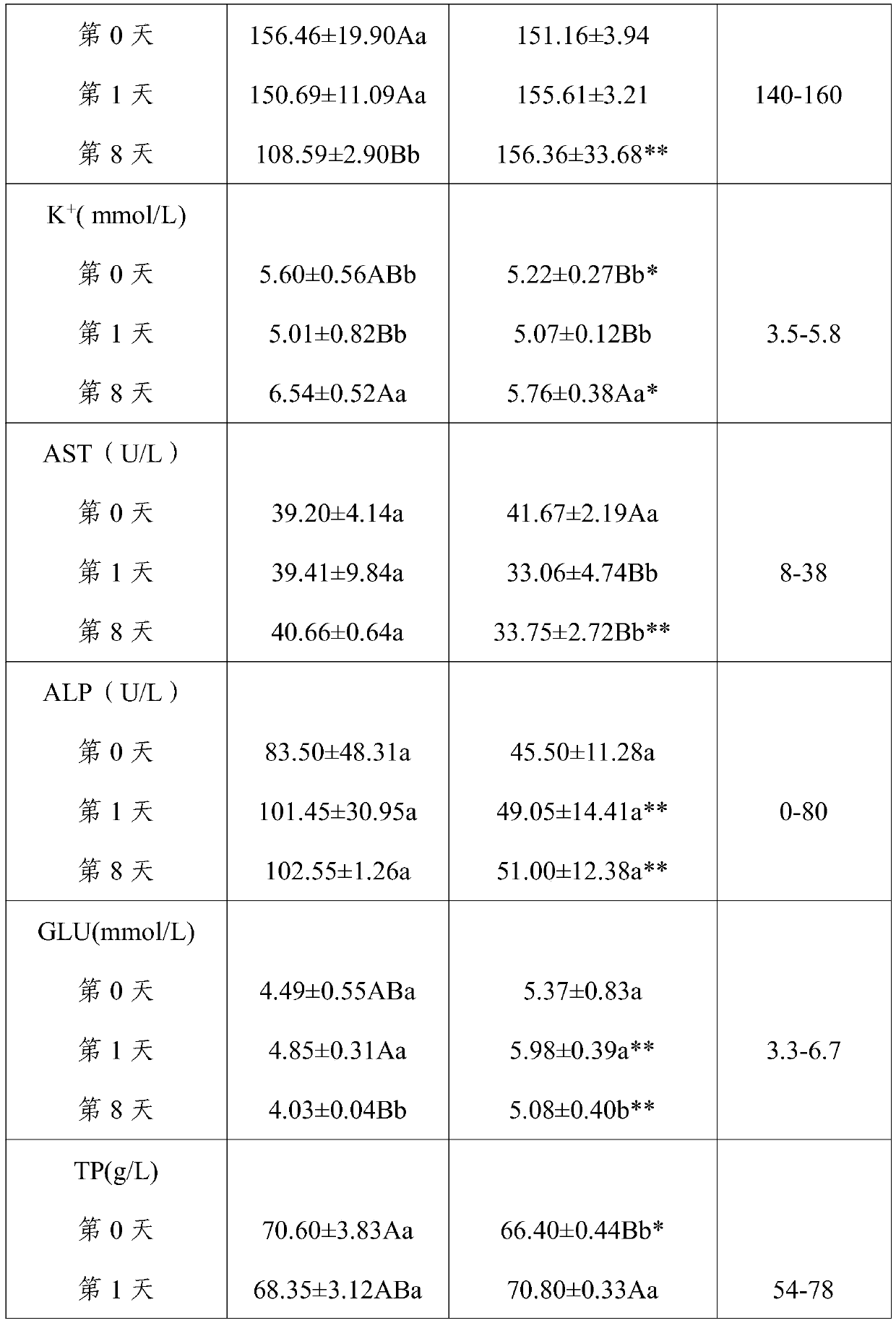
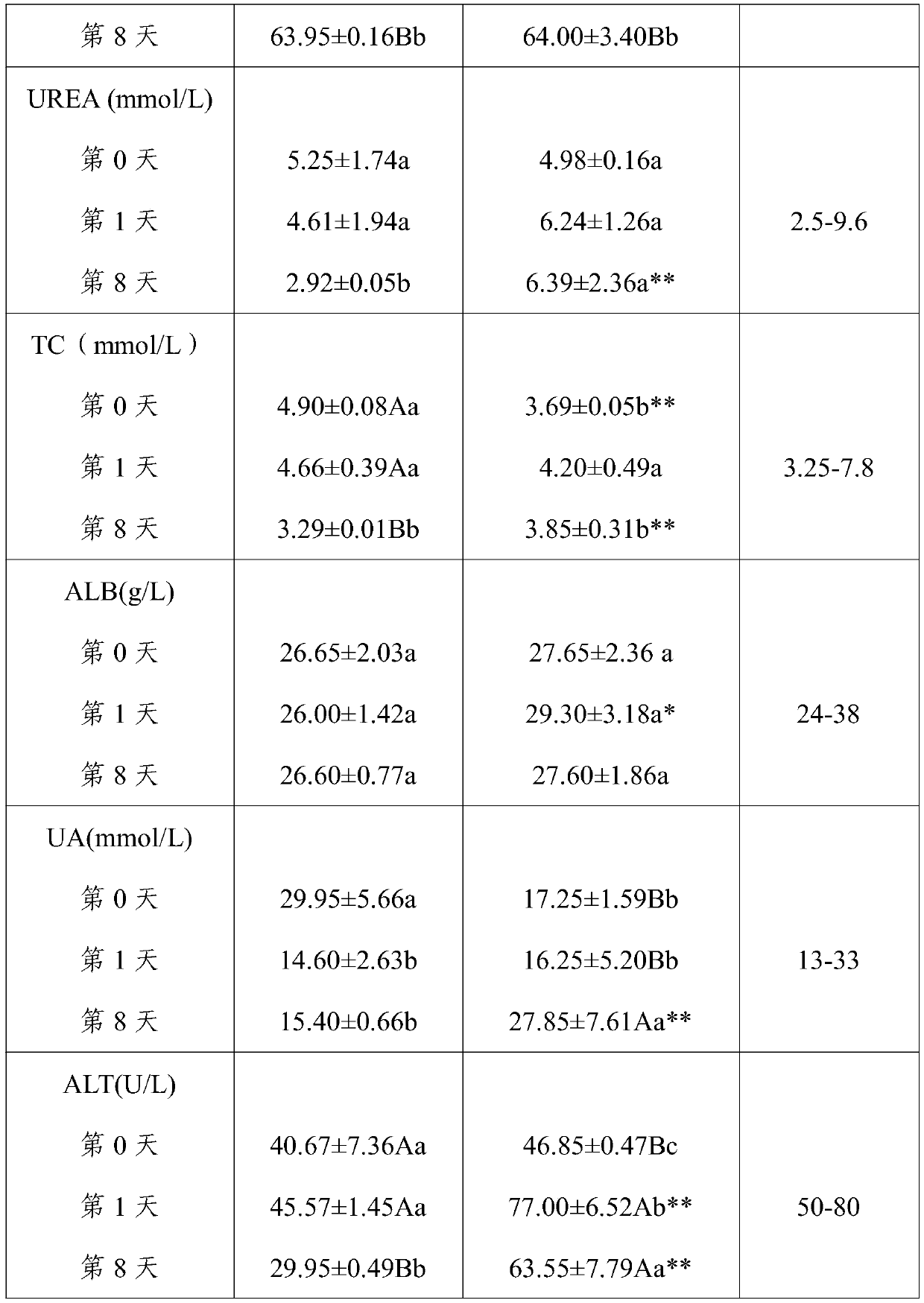
[0039] (1) Experimental animals: 12 adult healthy Chinese pastoral dogs, 6 males and 6 females. Immunization with pentavalent vaccine (produced by Wuxing Animal Health Pharmaceutical Factory in Jilin Province); deworming with ivermectin injection (Anhui Tianan Biotechnology Co., Ltd., approval number: Veterinary Medicine (2015) 120482645, license number: (2014) Veterinary Drug Production Certificate No. 12048). The test dogs were fed chicken oat dog food (produced by Xingtai Aopai Pet Food Co., Ltd.) for the whole period of time, once a day in the morning and evening, and drinking water was not restricted.
[0040] (2) Test method: 12 Chinese pastoral dogs were divided into control group and cataplasm administration group according to the principle of similar body weight, each group had three males and females, and all test dogs were fed with anhydrous s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com