Aminooxy structure-containing polymer and formaldehyde adsorbent, and preparation methods thereof
A formaldehyde adsorption and aminoxy technology, applied in chemical instruments and methods, separation methods, alkali metal compounds, etc., can solve the problems of non-renewable scavenging ability, unstable Schiff base, poor use effect, etc., and achieve excellent thermal stability Sexuality and chemical stability, excellent springback characteristics, and structural stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
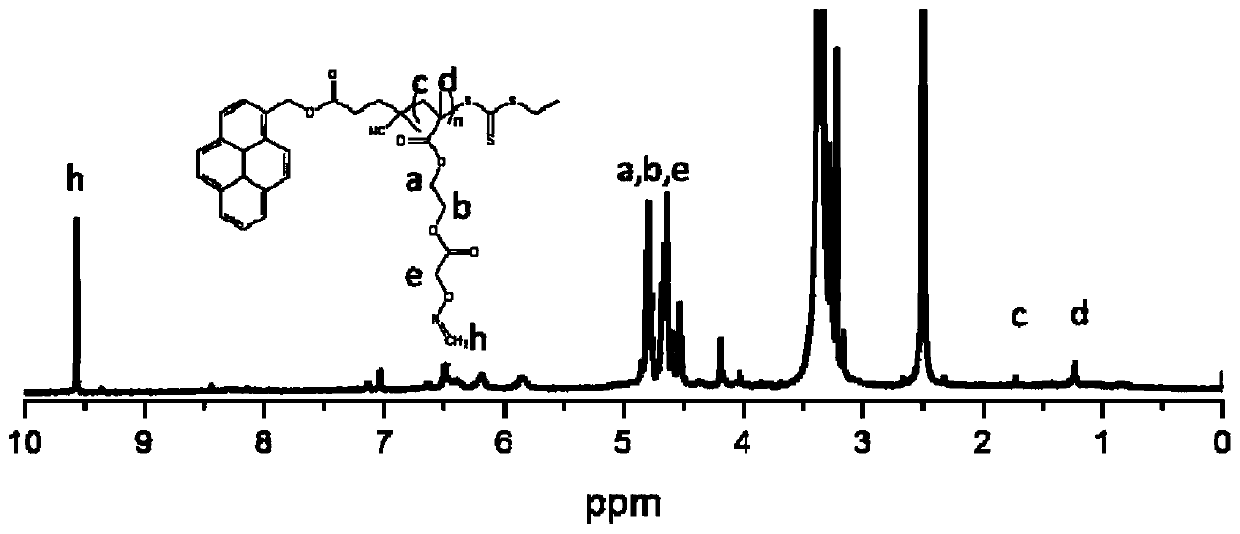
[0055] The RAFT polymerization of embodiment 1-1 methacrylic acid ethylene glycol acetate aminooxy ester monomer
[0056] This embodiment provides a kind of synthesis of poly(ethylene glycol acetate) aminooxy methacrylate, the end of the polymer has an aminooxy group, and its structural formula is as follows:
[0057]
[0058] The above-mentioned poly(ethylene glycol acetate) aminooxy methacrylate is prepared by RAFT polymerization method, comprising the following main steps:
[0059] a. Utilize the esterification reaction to synthesize the methacrylic acid ethylene glycol acetate aminooxy ester monomer containing aminooxy groups:
[0060] (1) if figure 1 As indicated, 0.8g of hydroxypropyl methacrylate, 1.0g of [(tert-butoxycarbonyl)aminooxy]acetic acid, 1.6g of N,N'-dicyclohexylcarbodiimide (DCC) and 100mg of 4-dimethyl Aminopyridine (DMAP) was dissolved in 10 mL of tetrahydrofuran in a 50 mL round bottom flask, and stirred at room temperature for 10 hours;
[0061] (2...
Embodiment 1-2
[0069] The ATRP polymerization of embodiment 1-2 p-aminooxystyrene monomer
[0070] This embodiment provides a synthesis of poly-p-aminooxystyrene, the end of the polymer has aminooxy groups, and its structural formula is as follows:
[0071]
[0072] The above-mentioned poly(p-aminooxystyrene) is prepared by ATRP polymerization method, including the following main steps:
[0073] (1) Dissolve 71mg of cuprous bromide and 98mg of bipyridyl in a 50mL round bottom flask with 10mL of toluene;
[0074] (2) feed nitrogen into the round bottom flask that solution is housed in step (1) and remove oxygen for 15 minutes;
[0075] (3) Dissolve 0.5g of p-aminooxystyrene and 0.2mL of ethyl bromoisobutyrate in a 50mL round bottom flask with 10mL of toluene;
[0076] (4) feed nitrogen into the round bottom flask that solution is housed in step (3) and remove oxygen in 15 minutes;
[0077] (5) Transfer the liquid in the round bottom flask in step (2) to the round bottom flask in step (4...
Embodiment 2-1
[0080] Embodiment 2-1 PHEMA, graphene oxide, melamine
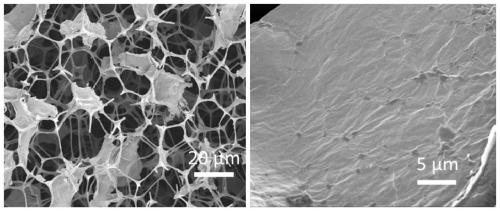
[0081] The present embodiment provides a kind of preparation method of regenerable formaldehyde adsorbent, comprises the following main steps:
[0082] (1) Synthesis of aminooxy-containing polymers
[0083] Adopt the method for embodiment 1-1 to prepare poly(ethylene glycol acetate aminooxy methacrylate) (PHEMA);
[0084] (2) Preparation of graphene polymer composite airgel
[0085] (1) 10 mg of graphene oxide and 10 mg of PHEMA synthesized in step (1) are dispersed in a 50 mL beaker with 10 mL of water;
[0086] (2) Ultrasonic treatment of the solution obtained in step (1) for 2 hours;
[0087] (3) Soak the cut melamine foam (30mm×30mm×5mm) into the solution obtained in step (2), and squeeze it repeatedly;
[0088] (4) Add 20 mg of ascorbic acid to the solution obtained in step (3), and react at 70° C. for 10 hours;
[0089] (5) The foam obtained after the reaction in step (4) is taken out from the solution, dialyze...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com