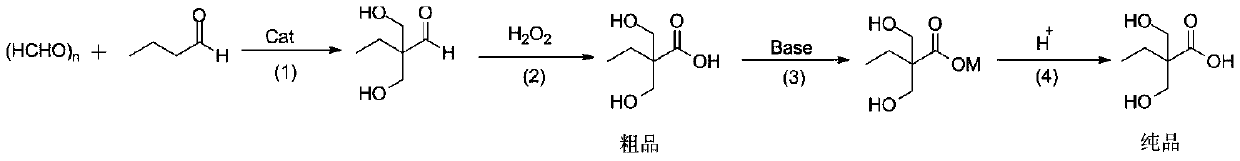
Preparation method of 2,2-dimethylolbutyric acid
A technology of dimethylolbutyric acid and dimethylolbutyraldehyde is applied in the field of preparing 2,2-dimethylolbutyric acid, and can solve the industrial production limitation and analysis of 2,2-dimethylolbutyric acid. The crystal process is unstable and the utilization rate of formaldehyde is low, so as to achieve the effect of improving the utilization rate of formaldehyde, easy separation and stable production process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 72g of butyraldehyde and 60g of paraformaldehyde into a 500mL three-necked flask equipped with a stirrer, reflux condenser and thermometer, start stirring and heating, preheat to 45°C, add dropwise 19.7g of 30% trimethyl gum aqueous solution, and control the reaction The temperature is 45-50°C, and the reaction is 60 minutes.
[0031] After the reaction, the catalyst and by-products were distilled off under reduced pressure at 60°C, and 113g of hydrogen peroxide was added dropwise under heating at 50°C, and then 40g of sodium hydroxide was added for neutralization at 70°C for 1 hour. The water was distilled off, and an appropriate amount of ethanol was added, the inorganic salt was removed by filtration, the pH of the mother liquor was adjusted to 4 with sulfuric acid, and the ethanol was removed to obtain 104.4 g of 2,2-dimethylolbutyric acid white crystal product with a purity of 99.5%. The yield was 70.5% (in terms of butyraldehyde, the same below).
Embodiment 2
[0033] Add 72g of butyraldehyde and 60g of paraformaldehyde into a 500mL three-necked flask equipped with a stirrer, reflux condenser and thermometer, start stirring and heating, preheat to 30°C, add 10.1g of triethylamine dropwise, and control the reaction temperature at 30°C. -40°C, react for 60 minutes.
[0034] After the reaction was over, the catalyst and by-products were removed by distillation under reduced pressure at 60°C, and 113g of hydrogen peroxide was added dropwise under heating conditions at 50°C, and then 56g of potassium hydroxide was added for neutralization after being kept at 70°C for 1 hour. Water was distilled off, and an appropriate amount of acetone was added, inorganic salts were removed by filtration, the pH of the mother liquor was adjusted to 4 with acetic acid, and the acetone was removed to obtain 108.1 g of 2,2-dimethylolbutyric acid white crystal product with a purity of 99.5%. The yield was 73.1%.
Embodiment 3
[0036] Add 72g of butyraldehyde and 75g of paraformaldehyde into a 500mL three-necked flask equipped with a stirrer, reflux condenser and thermometer, start stirring and heating, preheat to 30°C, add 4.4g of diethylamine dropwise, and control the reaction temperature at 30°C. ~40°C, react for 60 minutes.
[0037] After the reaction was over, the catalyst and by-products were removed by distillation under reduced pressure at 60°C, and 113g of hydrogen peroxide was added dropwise under heating conditions at 50°C, and then 56g of potassium hydroxide was added for neutralization after being kept at 70°C for 1 hour. Water was distilled off, and an appropriate amount of acetonitrile was added, inorganic salts were removed by filtration, the pH of the mother liquor was adjusted to 4 with phosphoric acid, and the acetonitrile was removed to obtain 111.2 g of 2,2-dimethylolbutyric acid white crystal product with a purity of 99.5%. The yield was 75.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com