Fluorine-containing silicon-containing polyurethane material with high biological stability and preparation method thereof
A technology of biological stability and polyurethane materials, which is applied in the field of fluorine-containing silicon-containing polyurethane materials and its preparation, can solve the problems that the biological stability and mechanical properties cannot be improved simultaneously, the mechanical properties of polyurethane are reduced, and the biological stability and mechanical properties of polyurethane materials are difficult. At the same time, problems such as guarantee are obtained, and the effect of improving the body and surface properties and improving biological stability is achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
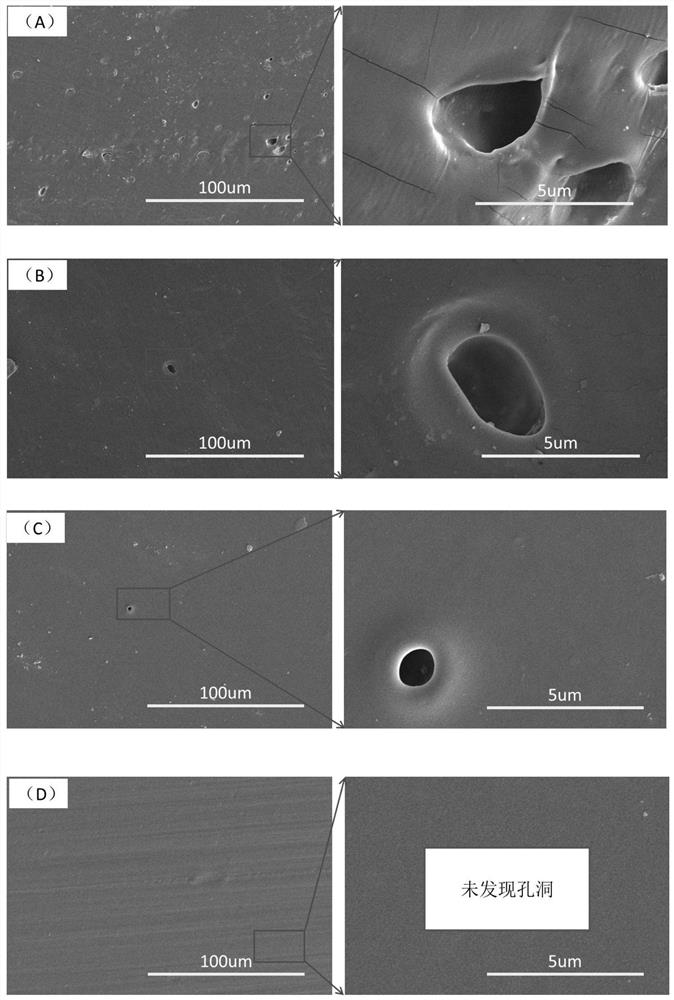
Examples
Embodiment 1
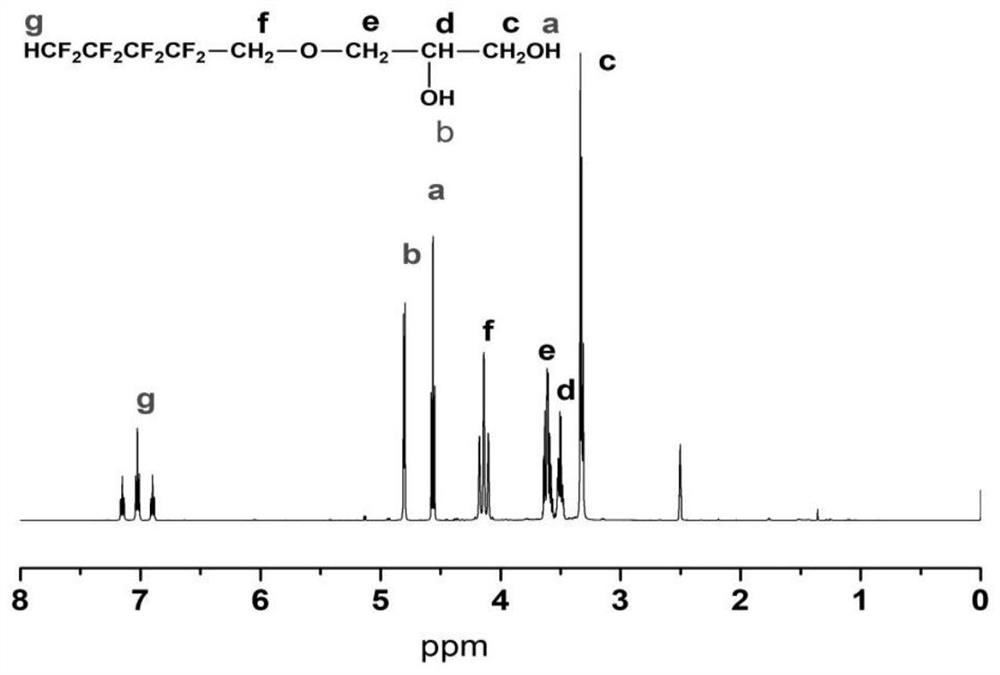
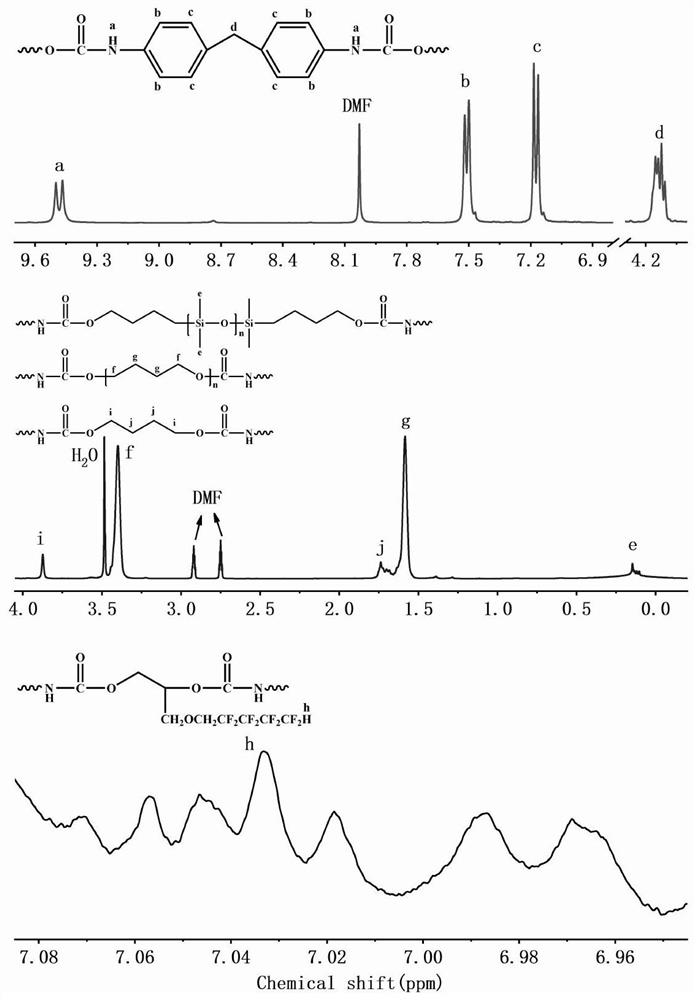
[0036] The first step, the preparation of fluorosilicone polyether polyurethane prepolymer, add 90g of polytetrahydrofuran ether diol and 22g of hydroxyalkyl-terminated polydimethylsiloxane into a three-necked bottle with mechanical stirring at 100-105°C Vacuum dehydration for 2 hours until the water content in the raw material is less than 0.01%; then blow in N 2And cool down to 60-65°C, add 3.06g FDO (fluorine-containing dihydric alcohol), stir evenly and continue to cool down to 40-42°C, add 51g of molten completely transparent liquid MDI (methylene diphenyl diisocyanate) and 0.1% organic bismuth catalyst, stir rapidly until the temperature rises to 68-70°C, and maintain the temperature for 50 minutes until the NCO (isocyanate group) content reaches the designed theoretical value, that is, the fluorosilicon polyether polyurethane prepolymer is obtained.
[0037] The second step is the post-chain extension reaction. Cool the prepolymer prepared in the first step to 58-60°C, ...
Embodiment 2
[0040] The first step, the preparation of fluorosilicon polyether polyurethane prepolymer, add 0.095mol polyhexamethylene ether glycol and 0.005mol hydroxyethoxyl hydrocarbon group-terminated polydimethylsiloxane into three Vacuum dehydrate the bottle at 100-105°C for 2 hours until the water content in the raw material is less than 0.01%; then blow N 2 And lower the temperature to 60-65°C, add 0.01molHEPFOA (N-(1,1-Dihydroxymethylethyl) perfluorooctylamide, see patent: fluorine-containing diol CN1435410A), stir well and continue to cool down to 40- 42°C, add 0.25mol HDI (hexamethylene 1,6-diisocyanate) and 0.05% stannous octoate, stir rapidly until the temperature rises to 75-80°C, and maintain the reaction at this temperature for 50min until the NCO content reaches Design the theoretical value to obtain the fluorosilicone polyether polyurethane prepolymer.
[0041] The second step is the post-chain extension reaction. Cool the prepolymer prepared in the first step to 58-60 °...
Embodiment 3
[0044] The first step, the preparation of fluorosilicone polycarbonate polyurethane prepolymer, add 0.005mol poly 1,6-hexyl carbonate diol and 0.095mol hydroxyalkyl terminated polydimethylsiloxane into three Vacuum dehydrate the bottle at 100-105°C for 2 hours until the water content in the raw material is less than 0.01%; then blow N 2 And cool down to 60-65°C, add 0.001mol FDO, stir evenly and continue to cool down to 40-42°C, add 0.204mol molten and completely transparent liquid MDI and 0.1% organic bismuth, stir rapidly until the temperature rises to 50-55°C , and maintain the temperature at this temperature for 50 minutes until the NCO content reaches the design theoretical value, that is, the fluorosilicon polycarbonate polyurethane prepolymer is obtained.
[0045] The second step is the post-chain extension reaction. Add 0.099mol BDO to the prepolymer prepared in the first step, stir evenly at a high speed and raise the temperature to about 100°C. When the viscosity of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com