PEG (polyethylene glycol)-polylysine/isothiocyanate bonding compound and application thereof as drug carrier
A technology of isothiocyanate and polyethylene glycol, which is applied in the field of anti-tumor nano-drugs, can solve the problems of poor stability of micelles and short half-life, and achieve enhanced anti-tumor effect, stability in vivo, and high drug loading rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Embodiment 1: the synthesis of polyethylene glycol-polylysine polymer
[0070] The synthetic method of polyethylene glycol-dendritic polylysine polymer is as follows:
[0071] N,N'-di-tert-butoxycarbonyl-L-lysine (Boc-Lys(Boc)-OH, 25g, 72.2mmol) and 2,3,4,5,6-pentafluorophenol (PFP, 15.9g , 86.6mmol) was dissolved in dioxane, and N,N'-dicyclohexylcarbodiimide (DCC, 17.8g, 86.6mmol) was added at 0°C, and the reaction solution gradually rose to room temperature, stirred overnight, iso Recrystallized in propyl ether to obtain white solid N,N'-di-tert-butoxycarbonyl-L-lysine pentafluorophenol ester (Boc-Lys(Boc)-OPFP, 28.6g);
[0072] Methoxy polyethylene glycol amino (PEG 5k -NH 2 , 1g, 0.2mmol), Boc-Lys(Boc)-OPFP (0.31g, 0.6mmol) and N,N-diisopropylethylamine (DIPEA, 0.2mL, 0.6mmol) were dissolved in 20mL dry dichloro In methane, stirred overnight under the protection of nitrogen at room temperature, concentrated the reaction solution, and precipitated in glacial ethe...
Embodiment 2
[0076] Embodiment 2: Preparation of polyethylene glycol-polylysine / phenylethyl isothiocyanate linkage
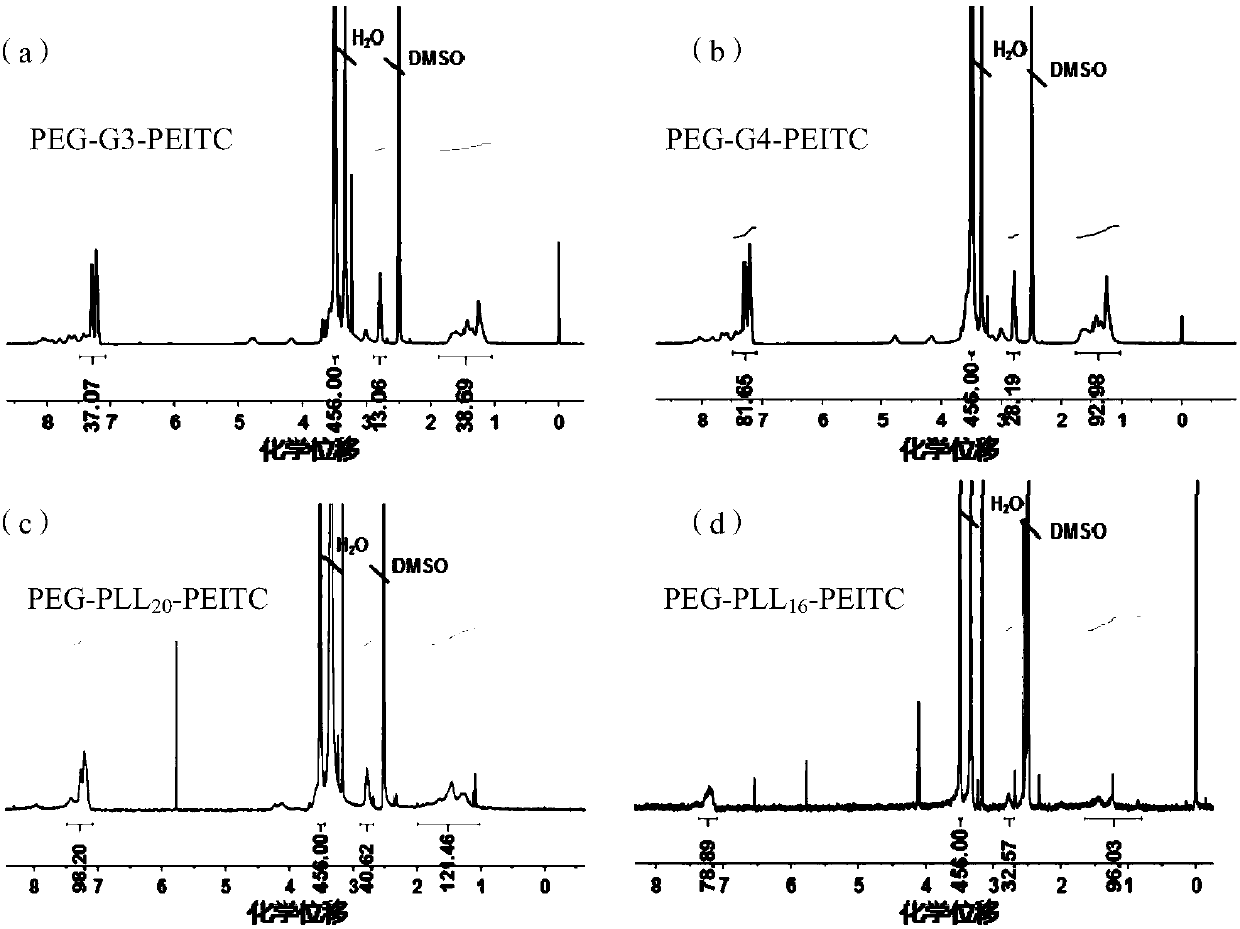
[0077] Taking the preparation of the third-generation polyethylene glycol-dendritic polylysine / phenylethyl isothiocyanate bond (PEG-G3-PEITC) as an example, the amino molarity of PEG-G3 and styrene The molar ratio of phenylethyl isothiocyanate is 1:1. Feeding: PEG-G3 (500mg, 0.073mmol) and phenylethyl isothiocyanate (PEITC, 100mg, 0.613mmol) are dissolved in 4mL of dimethyl sulfoxide , add 120 μL of triethylamine (Triethylamine, TEA, Mw=101.19), and react at 45° C. for 12 h. The reaction solution was put into a dialysis bag (molecular weight cut-off 3500), dialyzed against methanol for 24 hours, the methanol was removed by rotary evaporation under reduced pressure, and precipitated in ether to obtain a white powder, which was designated as PEG-G3-PEITC. Using deuterium band dimethyl sulfoxide as a solvent, the structure of the bond was characterized by NMR, 1 H NMR spectru...
Embodiment 3
[0084] Example 3: Polyethylene glycol-polylysine / phenylethyl isothiocyanate bonded paclitaxel
[0085] Encapsulation of anti-tumor drugs by thin film hydration method:
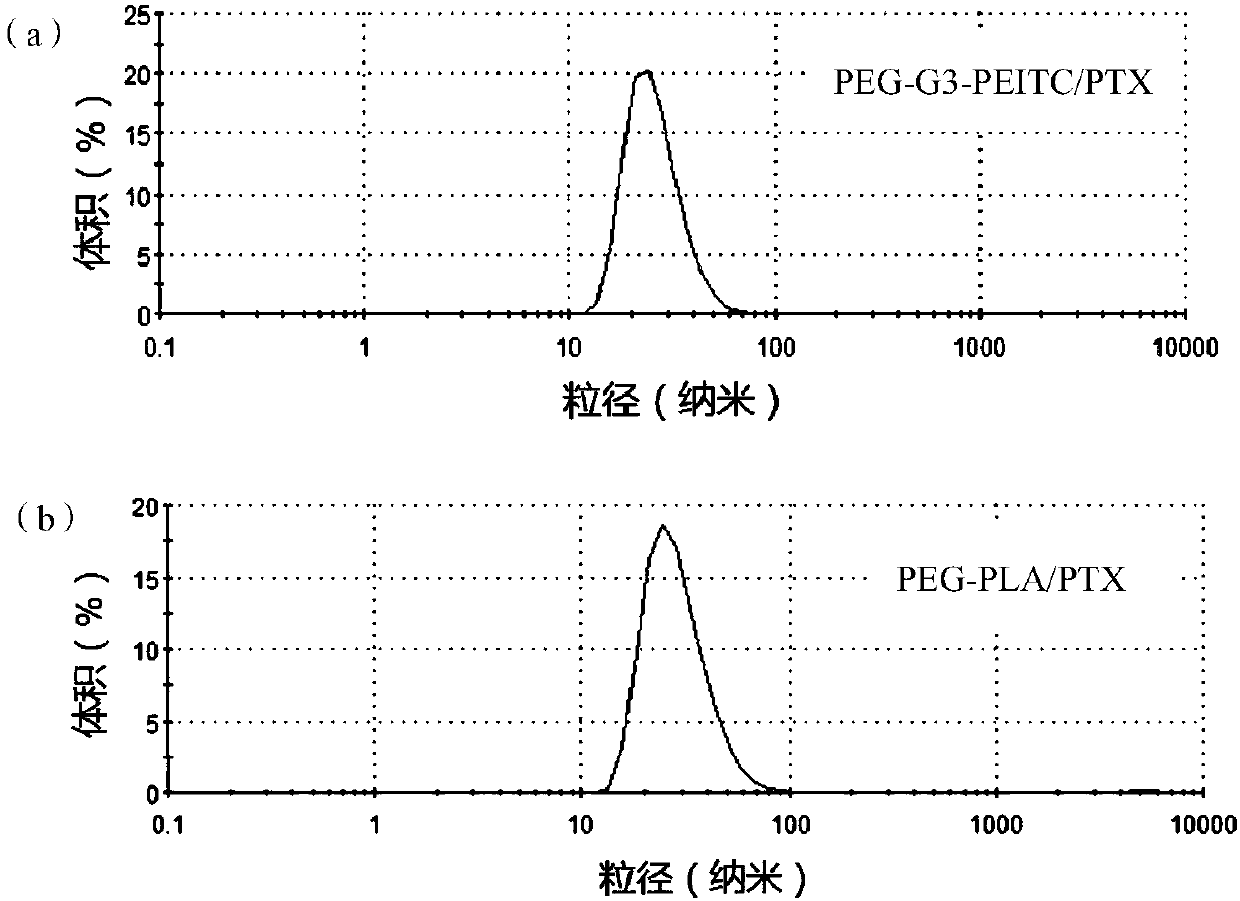
[0086] PEG-G3-PEITC (7.5 mg) and paclitaxel (2.5 mg) were dissolved in 1 mL of acetonitrile, and rotovaped at 40° C. under reduced pressure to form a film. Then, isothermal deionized water (2.5 mL) was slowly added dropwise while stirring to form nanomicelles, which were denoted as PEG-G3-PEITC / PTX, and its dynamic light scattering pattern in water (the content of PTX 1mg / mL) was as figure 2 As shown in (a), it can be seen from the figure that the particle size distribution of nano micelles is 0.130, the potential is -6.69mV, and the average size is 31.03nm. PEG-G4-PEITC-loaded paclitaxel micelles were prepared in the same way.
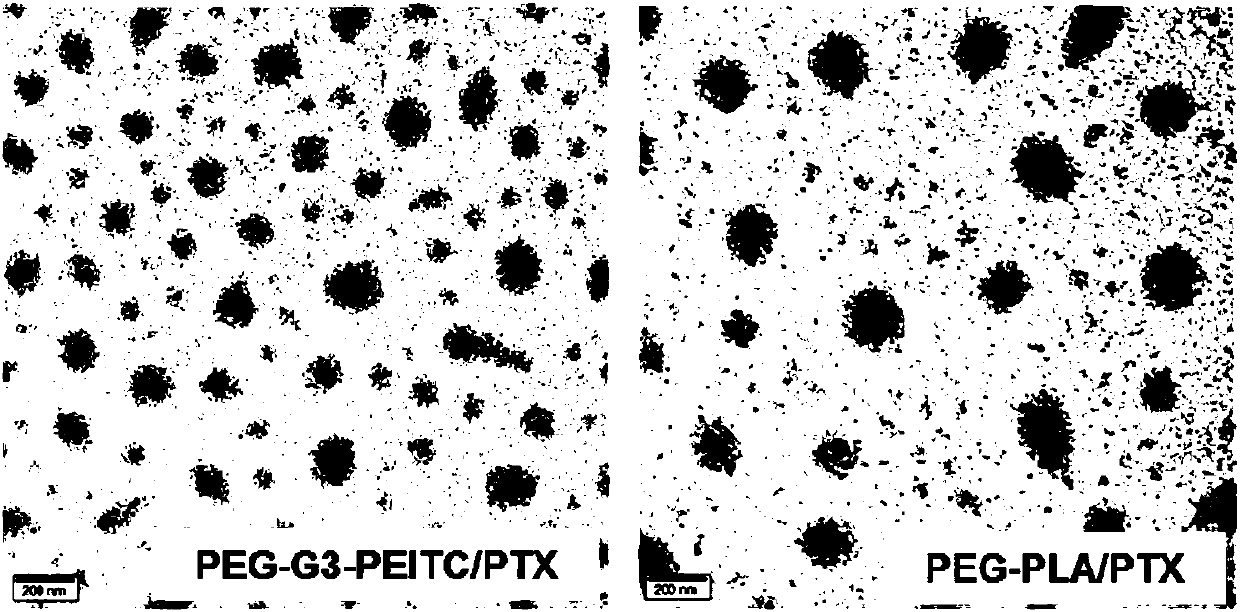
[0087] The morphology of PEG-G3-PEITC / PTX micelles was observed by transmission electron microscope (TEM). image 3 As shown in (left), spherical particles with a diameter of abou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com