Zinc chloride hydroxide having excellent zinc ion sustained-releasability and production method therefor
A hydroxide and manufacturing method technology, applied in the direction of zinc oxide/zinc hydroxide, zinc halide, medical preparations containing active ingredients, etc., can solve the problems of unstudied drug ingredients, etc., and achieve the effect of excellent stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0104] Hereinafter, although an Example demonstrates this invention concretely, this invention is not limited to an Example.
preparation example 1
[0106] 500 mL of a 0.08 M ammonium chloride aqueous solution was prepared in a reaction vessel, and 1000 mL of a 0.1 M zinc chloride aqueous solution was separately prepared as a dropwise reaction solution. A 30% by mass sodium hydroxide aqueous solution was prepared as a pH adjusting solution.
[0107] Using a pH controller connected to a pump, an aqueous zinc chloride solution and an aqueous sodium hydroxide solution were added dropwise while stirring while maintaining the pH of the above-mentioned ammonium chloride solution at 6.5. After all the zinc chloride aqueous solution was added dropwise, it was stirred for 16 hours and aged.
[0108]Thereafter, the reaction liquid was subjected to solid-liquid separation by centrifugation, and washing with water and centrifugation were repeated three times for the obtained solid. The precipitate washed in this way was vacuum-dried to obtain a dried zinzinite powder having a composition range represented by the formula (1).
preparation example 2
[0110] The pH at the time of synthesis was set at 5.5 to 10, and each sample obtained by changing the pH at the time of production including Production Example 1 (pH 6.5) was produced by the same process as Formulation Example 1. At pH 5.5, no precipitate was obtained because the pH was too low.
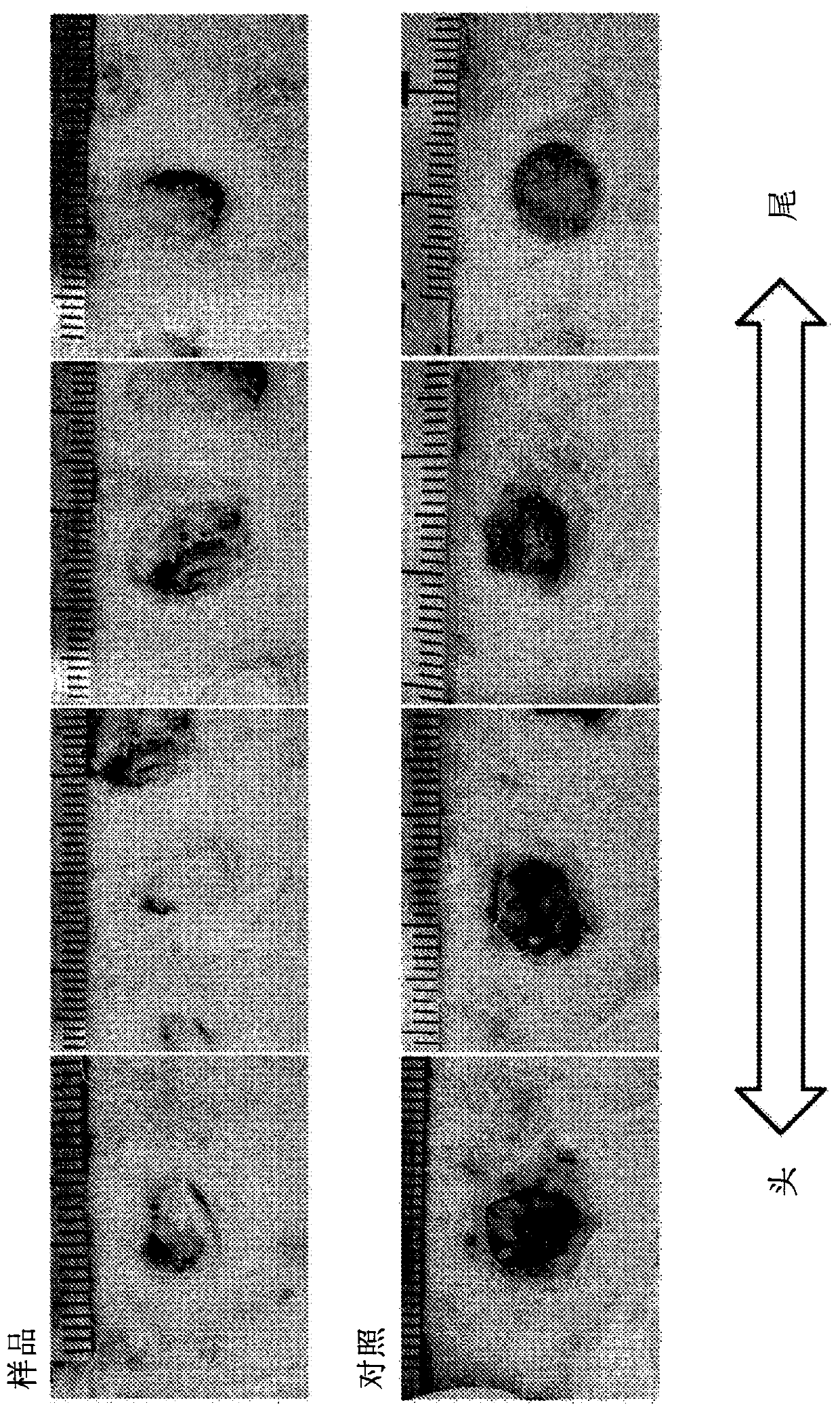
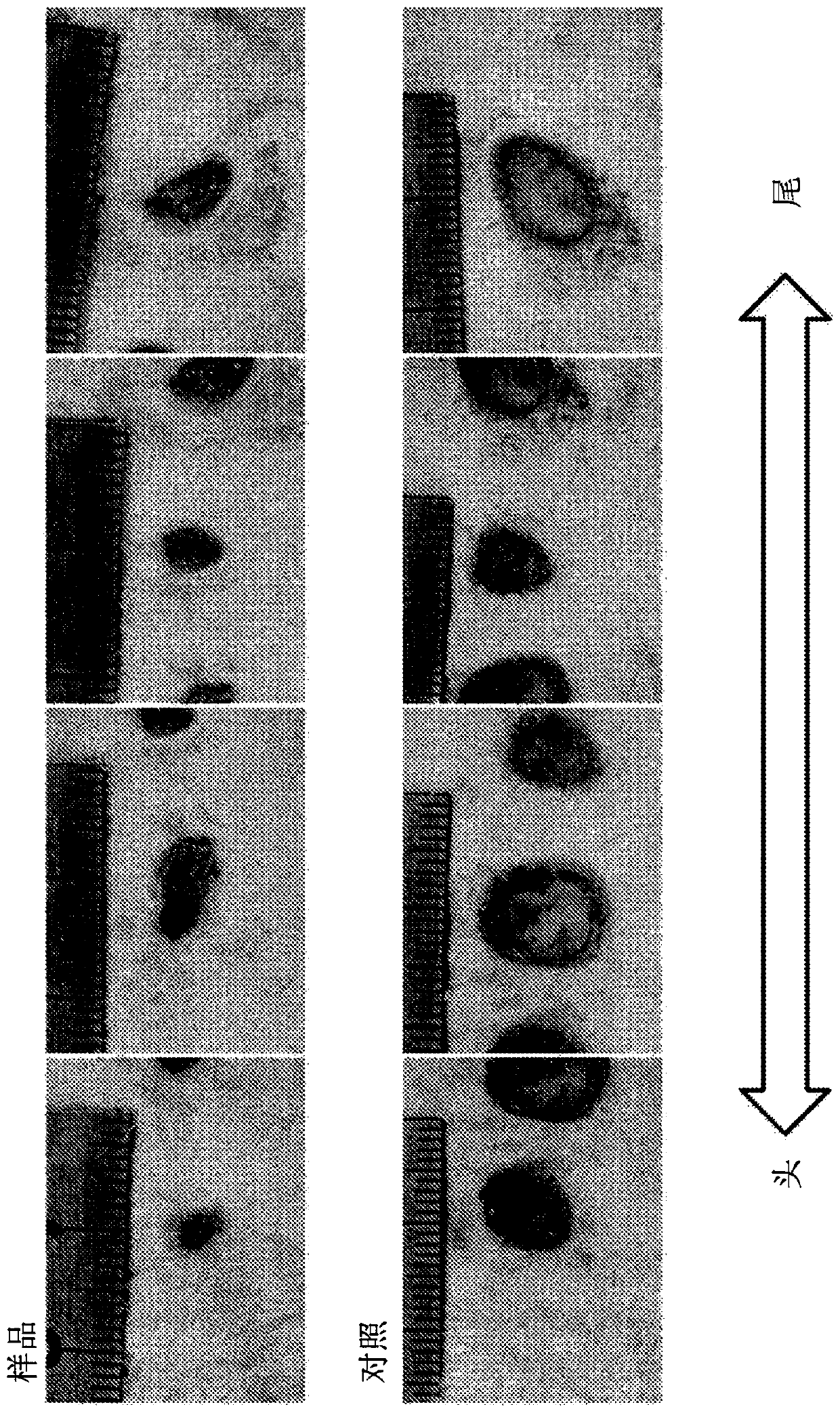
[0111] (dissolution test)
[0112] 0.6 g of each dry powder obtained in Formulation Example 2 was subjected to a dissolution test based on a stirring method using 30 g of physiological saline, and Zn 2+ Determination of ion dissolution and pH. For the dissolution test method, adjust the BET specific surface area of zinc chloride hydroxide hydrate containing chlorohydrinite to 10 to 150 m 2 / g, the mass ratio of zinc chloride hydroxide hydrate containing chlorohydrinite to normal saline is 1:50, the stirring time is 3 hours at 500 rpm using the rotor, and the pH and Zn after the dissolution test are measured 2+ Ion dissolution. show the result in Figure 7 , Figure 8 and Tabl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com