A kind of derivative of 17-hydroxysteroid, detection reagent and preparation method
A technology for detecting reagents and steroids, which is applied in the field of detection reagents and preparation of derivatives of 17-hydroxysteroids, and can solve problems such as poor stability and low sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
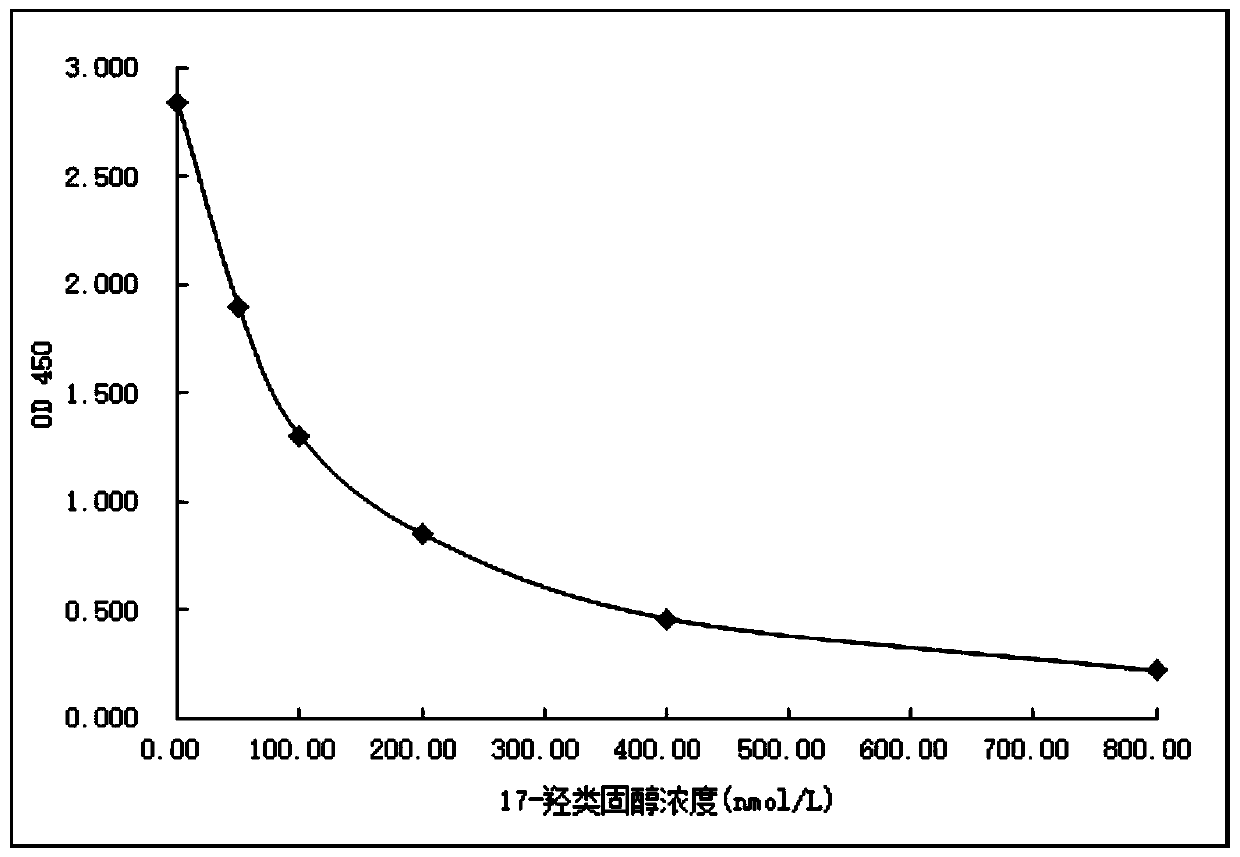
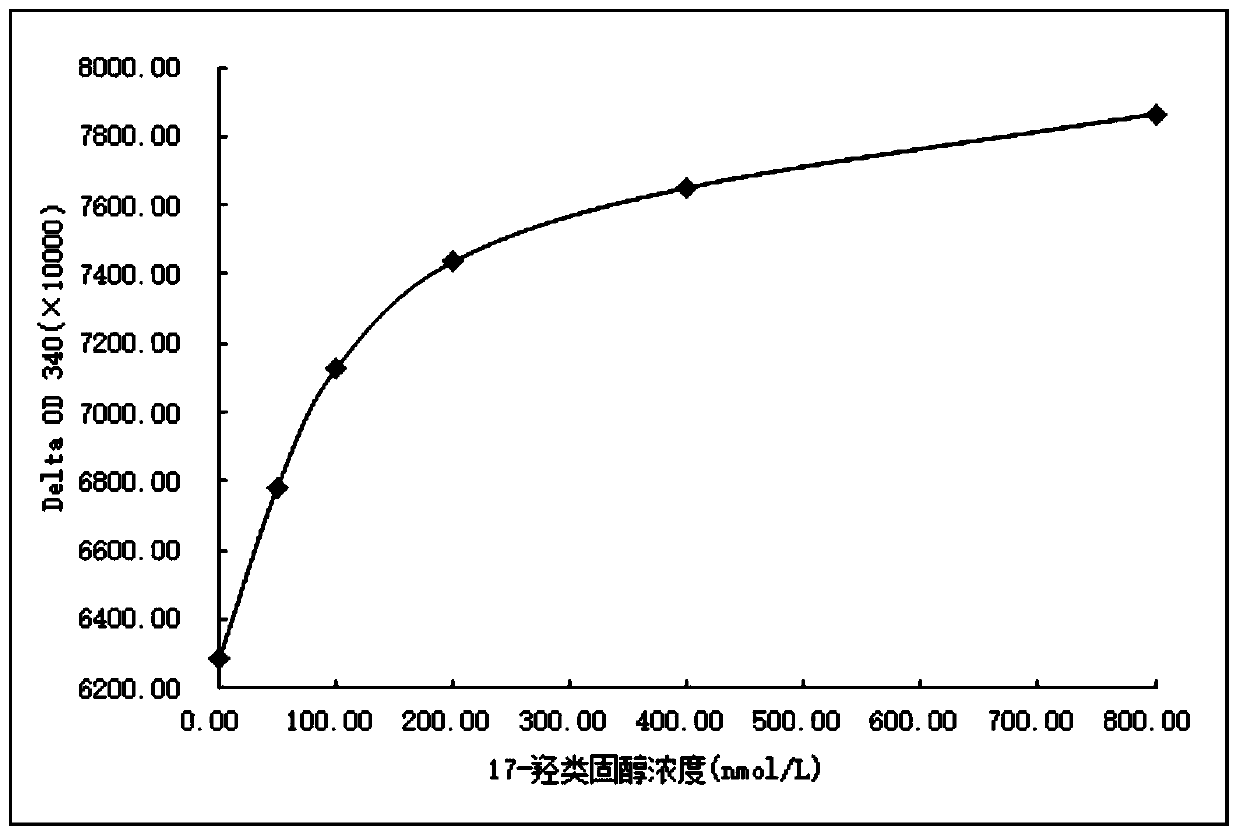
Image
Examples
Embodiment 1
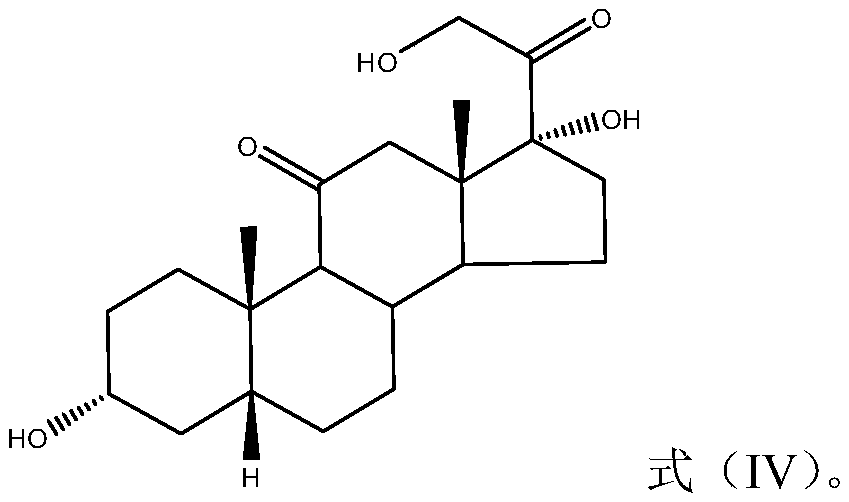
[0071] Example 1: Synthesis of 17-hydroxysteroid derivatives
[0072] The synthetic route of 17-hydroxysteroid derivatives is as follows:
[0073]
[0074] The preparation method of 17-hydroxy steroid derivatives, concrete steps are as follows:
[0075] (1) Synthesis of compound 2: Dissolve 5 g of compound 1 and 2.1 g of triethylamine (TEA) in dichloromethane (DCM) (50 mL), then add dihydrofuran-2,5- Diketone 1.8g, stirred at room temperature for 2 hours, thin layer chromatography (TLC) showed that after the reaction was complete, the reaction was quenched with 300mL purified water, the pH=3 was adjusted with 1N hydrochloric acid, and the reacted mixture was treated with 300mL DCM The extraction was repeated 3 times, the organic layers were combined, washed with purified water (200 mL) and brine (200 mL), dried and concentrated in vacuo to obtain 6 g of white solid, namely compound 2, yield: 94%;
[0076] (2) Synthesis of compound 3: Dissolve compound 2 (6g) obtained in s...
Embodiment 2
[0083] Example 2: Preparation of 17-hydroxysteroid immunogen and anti-17-hydroxysteroid specific antibody
[0084] The preparation steps of 17-hydroxysteroid immunogen are as follows:
[0085] (A1) Preparation of hemocyanin solution: 100 mg of hemocyanin was dissolved in 25 mL of 0.2 M, pH=8.5 phosphate buffer to obtain a hemocyanin solution;
[0086] (A2) Preparation of 17-hydroxysteroid derivative solution: 100 mg of the 17-hydroxysteroid derivative obtained in Example 1, 1.75 mL dimethylformamide, 1.75 mL ethanol, 3.5 mL, 10 mM, potassium phosphate at pH=5.0 Buffer, 100mg 1-ethyl-3-(-3-dimethylaminopropyl) carbodiimide, 25mg N-hydroxysulfosuccinimide were mixed, stirred and dissolved for 90min to obtain 17-hydroxysteroid derivatives solution;
[0087] (A3) Obtaining 17-hydroxysteroid immunogen: add the 17-hydroxysteroid derivative solution obtained in step (A2) to the hemocyanin solution obtained in step (A1), stir overnight at 4°C, and purify by dialysis , to obtain the 1...
Embodiment 3
[0092] Example 3: Preparation of 17-hydroxysteroid immunogen and anti-17-hydroxysteroid specific antibody
[0093] The preparation steps of 17-hydroxysteroid immunogen are as follows:
[0094] (A1) Preparation of thyroglobulin solution: 200 mg of thyroglobulin was dissolved in 50 mL, 0.2 M, pH=8.5 phosphate buffer to obtain thyroglobulin solution;
[0095] (A2) Preparation of 17-hydroxysteroid derivative solution: 200 mg of the 17-hydroxysteroid derivative obtained in Example 1, 3.5 mL of dimethylformamide, 3.5 mL of ethanol, 7.0 mL, 10 mM, potassium phosphate of pH=5.0 Buffer, 200mg 1-ethyl-3-(-3-dimethylaminopropyl) carbodiimide, 50mg N-hydroxysulfosuccinimide were mixed, stirred and dissolved for 90min to obtain 17-hydroxysteroid derivatives solution;
[0096] (A3) Obtaining 17-hydroxysteroid immunogen: add the 17-hydroxysteroid derivative solution obtained in step (A2) to the thyroglobulin solution obtained in step (A1), stir overnight at 4°C, and purify by dialysis , t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com