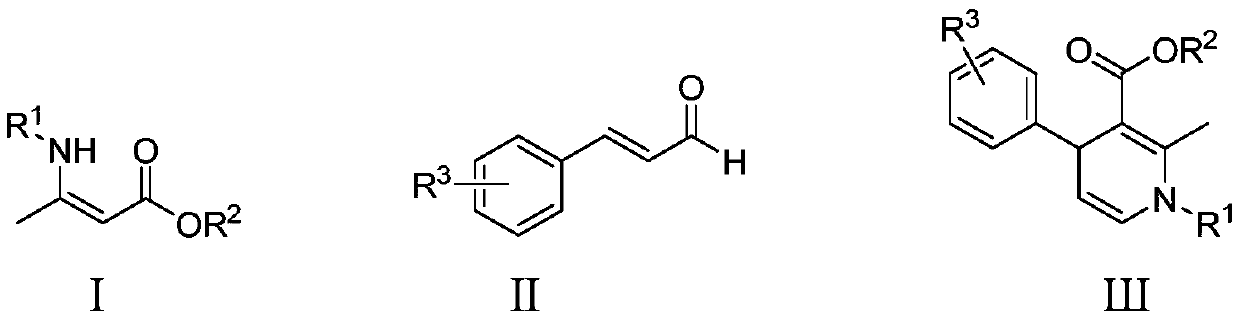
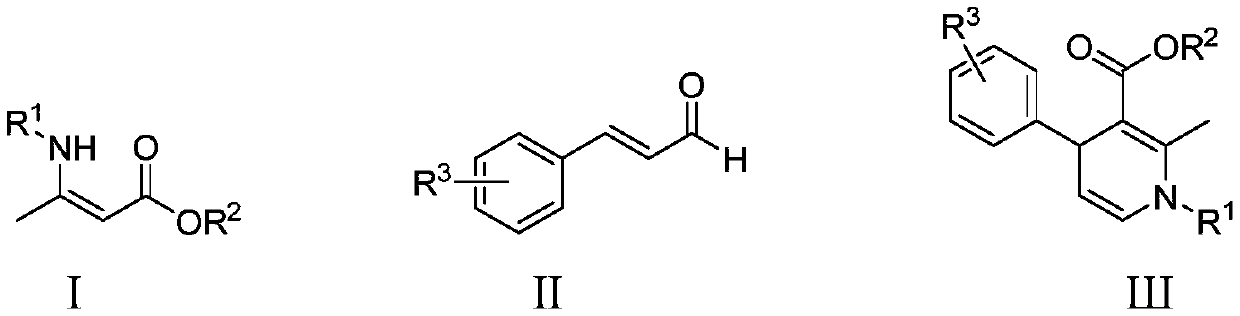
Method for catalytically synthesizing 1,4-dihydropyridine derivative through cooperation of zirconocene dichloride and benzoic acid ligands
A technology of zirconocene dichloride and benzoic acid, applied in the direction of organic chemistry, etc., to achieve the effects of mild reaction conditions, wide biological activity and medicinal value, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
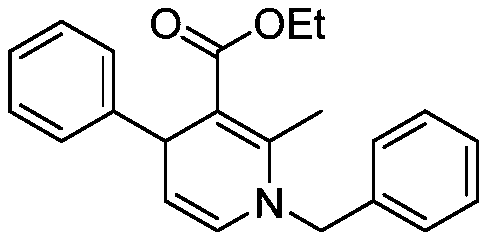
[0017] Preparation of ethyl 1-benzyl-2-methyl-4-phenyl-1,4-dihydropyridine-3-carboxylate of formula
[0018]
[0019] Into a 20mL reaction flask, add 0.2238g (1mmol) (Z)-3-(benzylamino)but-2-enoic acid ethyl ester, 146 μL (1.2mmol) cinnamaldehyde, 0.0125g (0.05mmol) dichlorodimethylene Zirconium, 0.0181g (0.1mmol) 5-nitrosalicylic acid, 3mL chloroform, stirred at 50 ° C for 2 hours, stopped the reaction, naturally lowered to room temperature, removed chloroform by rotary evaporation, and separated with a silica gel column ( The eluent is a mixture of ethyl acetate and petroleum ether in a volume ratio of 1:10) to obtain 1-benzyl-2-methyl-4-phenyl-1,4-dihydropyridine-3-carboxylic acid Ethyl ester, the yield was 96%, and the characterization data were: 1 H NMR (600MHz, CDCl 3 )δ7.35(t,J=7.5Hz,2H),7.27(dd,J=14.7,5.9Hz,5H),7.22(d,J=7.4Hz,2H),7.17-7.13(m,1H), 5.95(d, J=7.6Hz, 1H), 4.97(dd, J=7.6, 5.5Hz, 1H), 4.68-4.54(m, 3H), 3.97(q, J=7.1Hz, 2H), 2.41(s , 3H), 1.08(t, J=7.1...
Embodiment 2
[0021] In this example, equimolar 3-nitrophthalic acid was used to replace 5-nitrosalicylic acid in Example 1, and other steps were the same as in Example 1 to obtain 1-benzyl-2-methyl-4 - ethyl phenyl-1,4-dihydropyridine-3-carboxylate in 84% yield.
Embodiment 3
[0023] In this example, equimolar 4-nitrophthalic acid was used to replace 5-nitrosalicylic acid in Example 1, and other steps were the same as in Example 1 to obtain 1-benzyl-2-methyl-4 - ethyl phenyl-1,4-dihydropyridine-3-carboxylate in 90% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com