Malononitrile derivative near-infrared hydrogen sulfide fluorescent probe as well as preparation method and application thereof
A fluorescent probe, malononitrile technology, applied in chemical instruments and methods, sulfonate ester preparation, fluorescence/phosphorescence, etc., can solve the problems of high detection limit, large background interference, slow response speed of hydrogen sulfide, etc., and achieve synthesis The effect of short route, high sensitivity and excellent selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
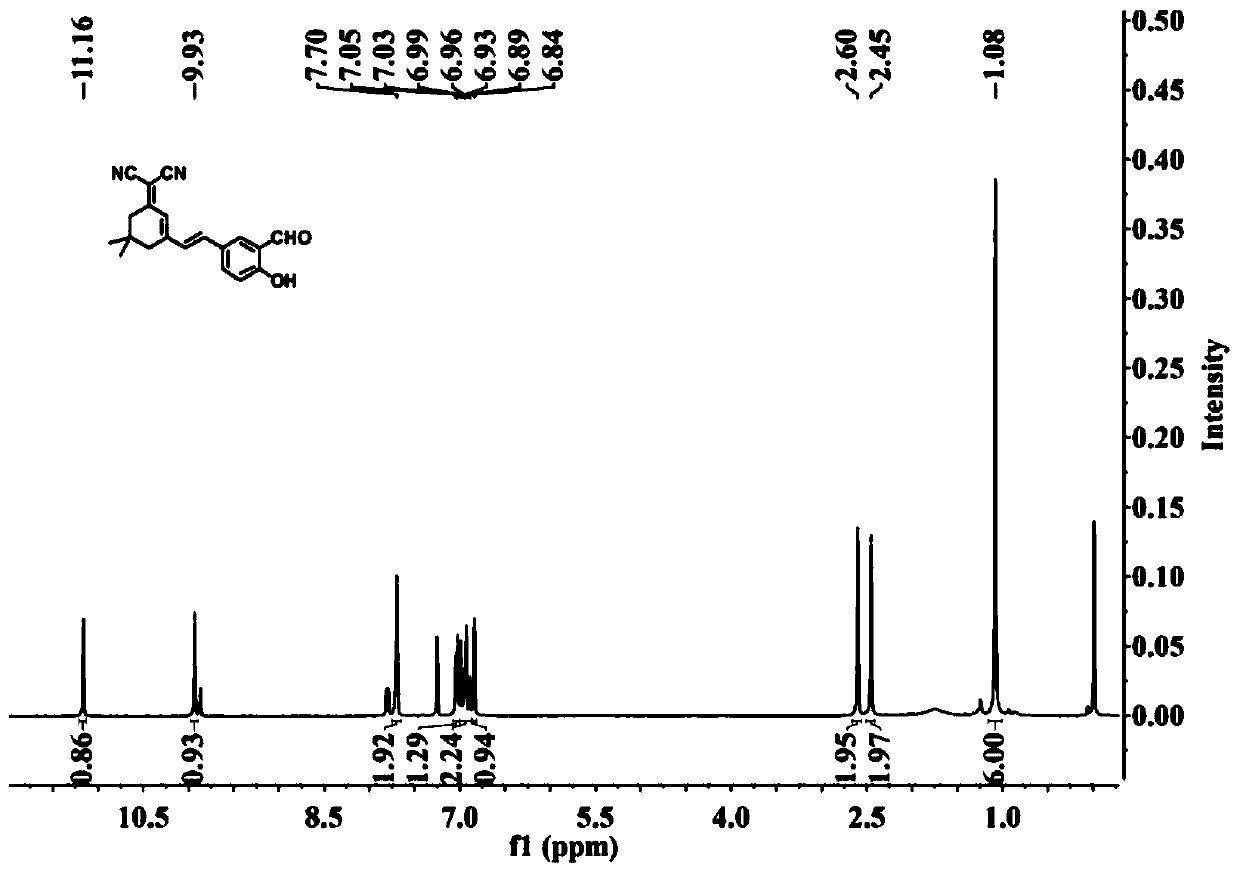
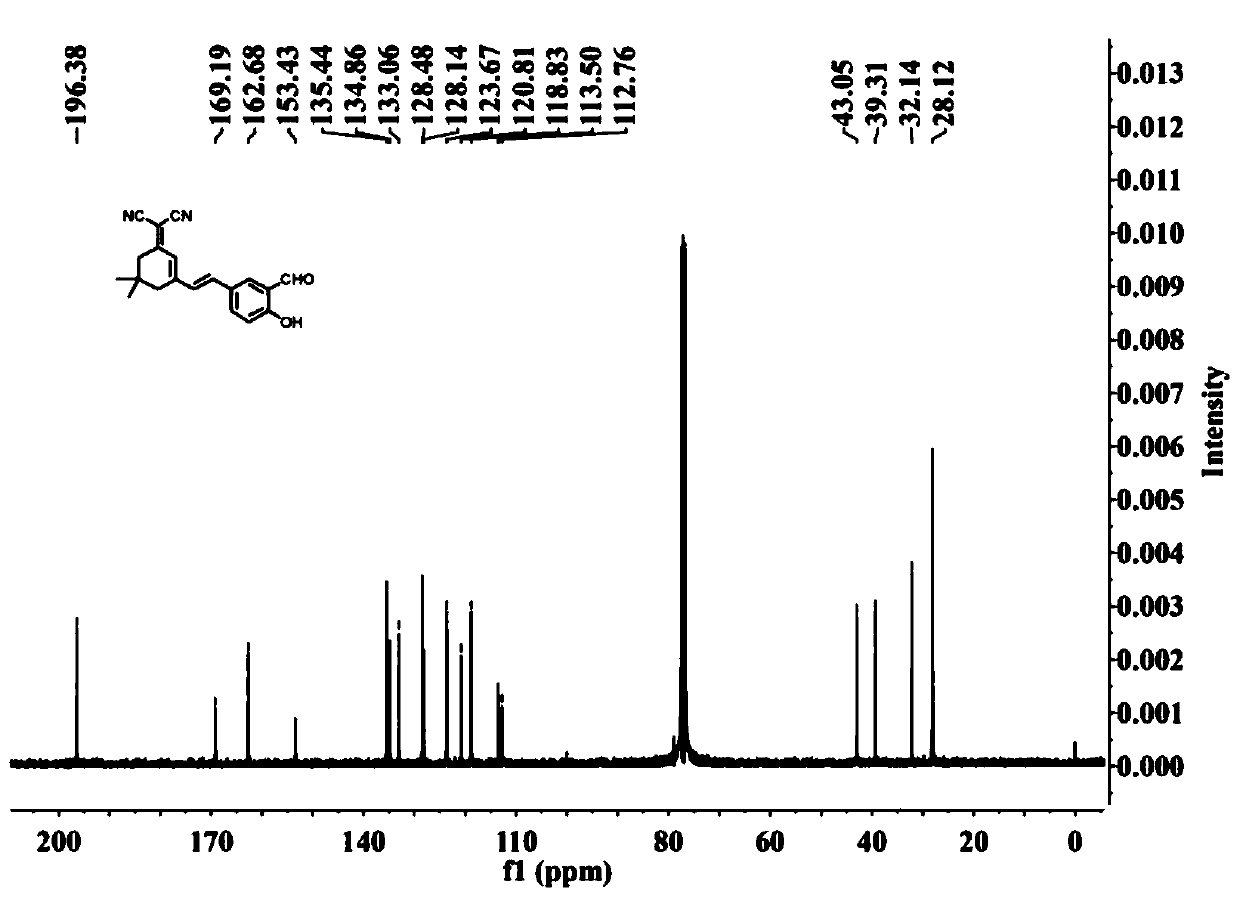
[0043] Embodiment 1 Preparation of malononitrile derivatives near-infrared hydrogen sulfide fluorescent probe
[0044] (1) Preparation of formula II compound
[0045]
[0046]In a 50mL reaction flask, add malononitrile (1.90g, 28.76mmol), absolute ethanol (30mL) and piperidine (1.22g, 14.38mmol) successively, react for 5-10min, add isophorone (2.00g, 14.38mmol), at N 2 Under protection, the reaction was carried out at 65°C, monitored by TLC (petroleum ether: ethyl acetate = 5:1, V / V), until the reaction was completed. After 8 hours, the reaction was completed. The reaction solution was cooled to room temperature, poured into 80 mL of ice water, stirred, and a solid precipitated out, filtered with suction, dried, and recrystallized from n-hexane to obtain 1.8 g of an off-white solid, which was the pure product of the compound of formula II. was 66.6%.
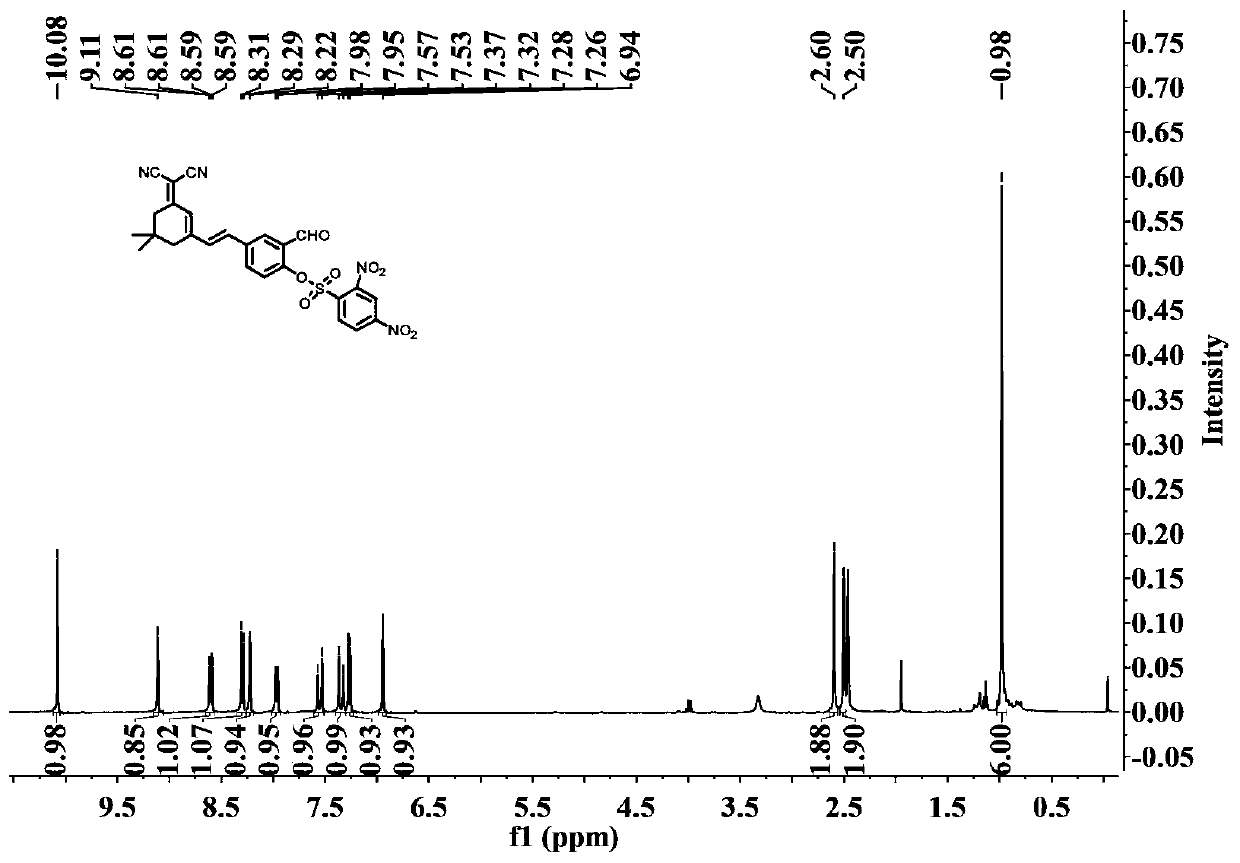
[0047] (2) preparation of formula III compound
[0048]
[0049] In the 50mL reaction flask, add formula II compound...
Embodiment 2
[0060] Embodiment 2 optical performance detection method
[0061] (1) Solution preparation required for optical performance testing
[0062] Preparation of fluorescent mother nucleus storage solution: Use an analytical balance of 1 / 100,000 to weigh 3.2mg of fluorescent mother nucleus, pour it into a 10mL volumetric flask, dilute it with DMSO, and prepare it into a fluorescent mother nucleus with a concentration of 1.0mmol Store the solution and store it in the refrigerator at low temperature and away from light.
[0063] Preparation of the storage solution of the fluorescent probe: Weigh 5.5 mg of the fluorescent probe with an analytical balance of 1 / 100,000, pour it into a 10 mL volumetric flask, dilute it with DMSO, and prepare it into a fluorescent probe with a concentration of 1.0 mmol. Needle storage solution, and store it in the refrigerator at low temperature and avoid light.
[0064] Preparation of storage solutions for other interfering biological substances: L-cyst...
Embodiment 3
[0087] The cytotoxicity experiment (MTT method) of embodiment 3 probe SFP-CHO-P
[0088] In order to ensure that the probe SFP-CHO-P is used in tissue imaging and detection of tissue hydrogen sulfide, this application uses the MTT method to detect the toxicity of the probe in terms of cell (HT22). Firstly, prepare for the experiment, dilute the probe SFP-CHO-P into different concentrations, keep it for later use, and set 3 (or more) duplicate wells for each concentration. HT22 cells (provided by the Neurobiology Research Center of Xuzhou Medical University) were seeded in 96-well plates, with 50,000 cells per well, and the culture conditions were 5% CO 2 , 37°C. When the HT22 cells grew to a suitable density, the probes of different concentrations prepared in advance were added to the wells and incubated for 24 hours. After incubation, add 10 μL of MTT solution (concentration: 5 g / mL) to each well for incubation (4-5 h), when the incubation is over, remove the culture medium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com