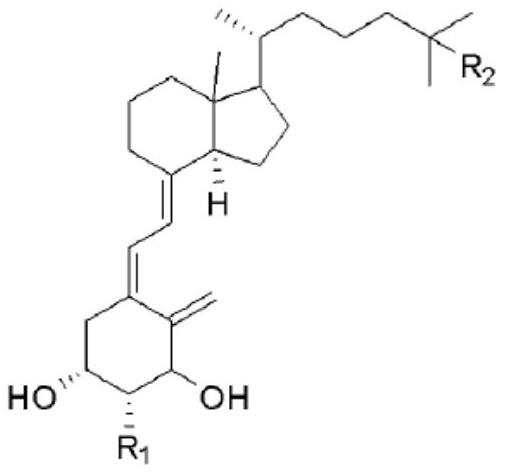
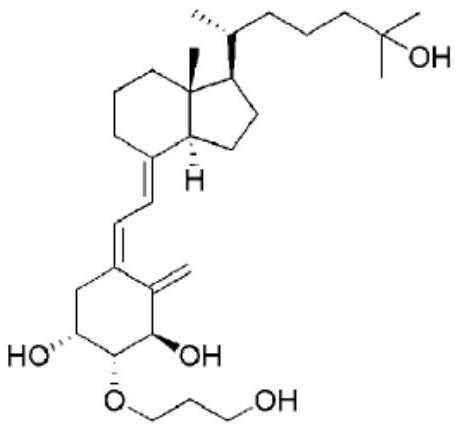
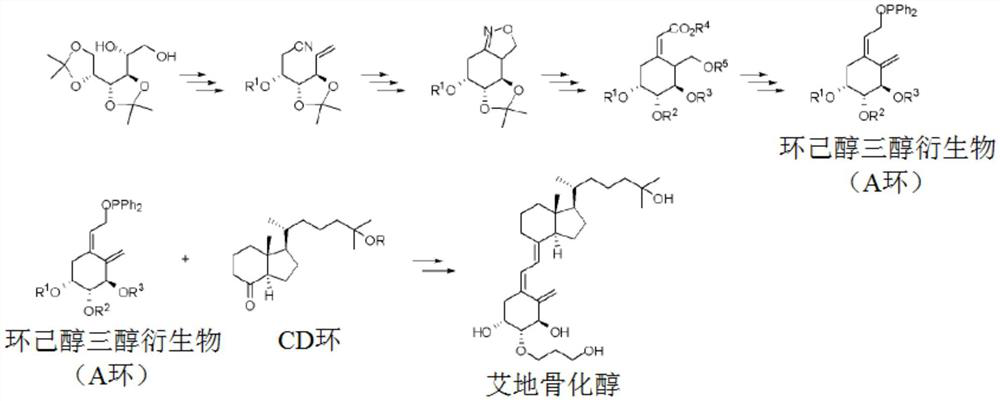
Preparation method of idecalcidol and intermediate used therefor
A technology of idecalcitol, chemical formula, applied in the field of a method and its intermediates, capable of solving problems such as long preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0224] Embodiment 1: the compound of preparation chemical formula 3
[0225] 503 g of (3R,4R)-hexa-1,5-diene-3,4-diol 2 were diluted in 5000 ml of cyclohexane. 2027 ml of p-anisaldehyde and 1170 g of p-toluenesulfonic acid were added, and the temperature of the reaction liquid was raised to about 45 to 50° C. and then stirred for four hours. The progress of the reaction was observed by thin layer chromatography (ethane:ethyl acetate=2:1). After the reaction was completed, the reaction solution was cooled to room temperature, and stirred after adding 3000 ml of 5% aqueous sodium bicarbonate solution. The organic layer was separated, and 6940 ml of 10% sodium bisulfite was added, followed by stirring. The organic layer was separated, and 1780 ml of a 5% aqueous sodium bicarbonate solution was added, followed by stirring. The organic layer was separated and dried over anhydrous sodium sulfate. After filtering anhydrous sodium sulfate, (4R,5R)-2-(4-methoxyphenyl)-4,5-divinyl-1...
Embodiment 2
[0227] Embodiment 2: the compound of preparation chemical formula 4
[0228] 200 g of (4R,5R)-2-(4-methoxyphenyl)-4,5-divinyl-1,3-dioxolane 3 containing impurities was diluted in 1800 ml of toluene. The temperature of the reaction liquid was cooled to about -5 to 0°C, and 1552 ml of diisobutylaluminum hydride (DIBAL, 1.2M in toluene) was added dropwise while maintaining 0°C. The reaction solution was stirred at the same temperature for about 1 to 2 hours, and the progress of the reaction was observed by thin layer chromatography (hexane:ethyl acetate=2:1). After completion of the reaction, 50.3 ml of methanol was dropped into the reaction solution to complete the reaction, and 50.3 ml of 5% NaOH aqueous solution was added dropwise while maintaining 10° C. or lower. After stirring the reaction for about 10 to 20 minutes, the organic layer was separated and dried over anhydrous sodium sulfate. After filtering anhydrous sodium sulfate, the mixture was obtained by concentrating ...
Embodiment 3
[0230] Embodiment 3: the compound of preparation chemical formula 5
[0231] Add 80g of molecular sieve (molecular sieve) to 960ml of dichloromethane 245ml of isopropyl titanate and 203ml of (+)-diisopropyl tartrate were stirred at room temperature for about 20 minutes. 162g of (3R,4R)-4-(4-methoxybenzyloxy)hexa-1,5-dien-3-ol 4 was diluted and added dropwise to 640ml of dichloromethane and stirred at room temperature for about 20 minutes. While maintaining below 20°C, 248ml of tert-butyl hydroperoxide was added dropwise and stirred at room temperature for about 5 hours, and the progress of the reaction was observed by thin layer chromatography (hexane:ethyl acetate=2:1). After the reaction, diatomaceous earth (celite) was used to filter and remove undissolved solids, and 230 g of ferrous sulfate heptahydrate and 79.5 g of citric acid were dissolved in 2400 ml of water and added to the filtrate. The reaction mixture was stirred at room temperature for 1 hour, and the organic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com