Detector combined method for measuring impurities of repaglinide in repaglinide metformin tablets
A metformin tablet and detector technology, which is applied in the direction of instruments, measuring devices, scientific instruments, etc., can solve the problems that the response cannot be completely eliminated, the accuracy and precision are affected, and the specificity of the method is reduced.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Instrument: Agilent1260 high performance liquid chromatography (G1329B autosampler, G1316A constant temperature column oven, G1311C quaternary pump, G4212B diode array detector, G1321B fluorescence detector).
[0046] Chromatographic column: AgelaVENUSIL ASB C18 chromatographic column (4.6mm×250mm, 5μm).
[0047] Mobile phase: 0.4% potassium dihydrogen phosphate buffer solution (adjusted to pH 3.2 with phosphoric acid) as mobile phase A and acetonitrile as mobile phase B, using gradient elution for separation. Gradient elution was performed according to Table 2.
[0048] Table 2 gradient elution table (volume ratio) / %
[0049]
[0050] Detector: diode array and fluorescence detector connected in series, the detection wavelength is 240nm, the excitation wavelength is 244nm, and the emission wavelength is 348nm.
[0051] Flow rate: 1.0ml / min, injection volume: 50μl, column temperature: 40°C.
[0052] Sample solution preparation diluent: ethanol-water (volume ratio 1...
Embodiment 2
[0058] Embodiment 2 specificity test
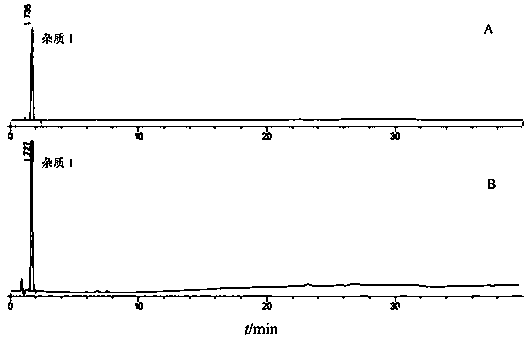
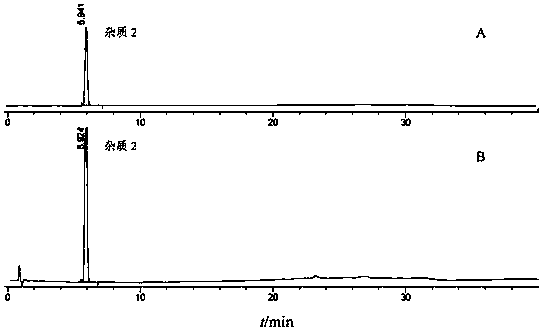
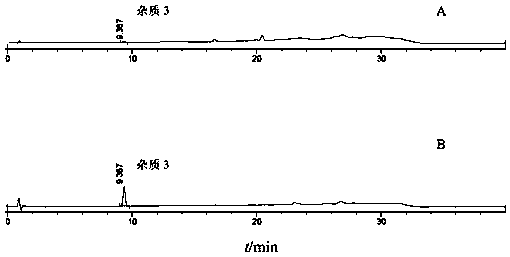
[0059] In the present invention, the compound repaglinide metformin tablet has been subjected to a forced destruction degradation test, which is destroyed by means of strong light irradiation, high temperature, acid, alkali hydrolysis and oxidation to study the separation degree of degradation impurities and main peaks, and detects in a diode array The peak purity of the main peak was verified in the instrument to prove the specificity of the method. Take 20 tablets of compound repaglinide metformin tablets, grind them into powder, weigh the tablet powders to prepare the following solutions.
[0060] a. Sample solution: Weigh an appropriate amount of tablet powder (approximately equivalent to 0.5mg repaglinide), put it in a 50ml measuring bottle, add diluent to dissolve repaglinide and reach the scale, shake well, filter, and take the continued filtrate as a sample solution.
[0061] b. Acid-destroyed sample solution: Weigh an appropria...
Embodiment 3
[0070] Embodiment 3 detection limit test
[0071] Take an appropriate amount of repaglinide and its impurities 1, 2, 3, 4, and 5, weigh them accurately, dissolve them with a diluent and dilute them into a series of solutions, inject samples for determination, and take the concentration point with a signal-to-noise ratio ≥ 3 As the limit of detection, the result is:
[0072] The detection limits of repaglinide, impurity 1, impurity 2, impurity 3, impurity 4, and impurity 5 were 0.06ng, 0.07ng, 0.05ng, 0.2ng, 0.1ng, and 0.06ng, respectively, and the detection limits of each impurity were within the main component 0.05 % concentration below, to meet the impurity detection sensitivity requirements.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com