Method for preparing bis(1-alkoxy-2,2,6,6-tetramethyl piperidine-4-yl) sebate
A technology of tetramethylpiperidine and tetramethylpiperidinol, applied in bis(1-alkoxy-2,2,6,6-tetramethylpiperidin-4-yl) sebacate In the field of preparation, it can solve the problems of by-products that are difficult to handle, difficult to handle, and pollute the environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
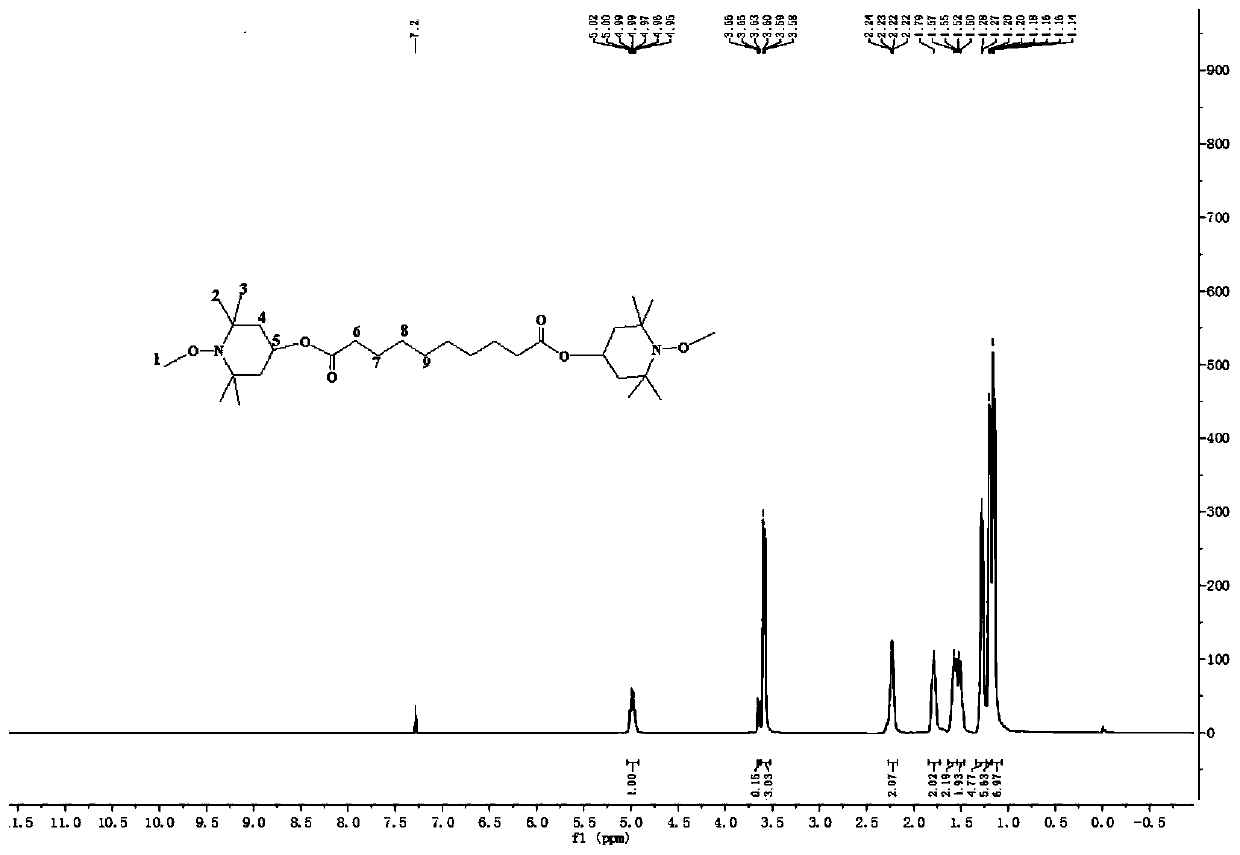
[0059] A preparation method of bis(1-methoxy-2,2,6,6-tetramethylpiperidin-4-yl) sebacate, comprising the steps of:
[0060] (1) Synthesis: 0.11mol (20.60g) of 1-methoxy-4-hydroxyl-2,2,6,6-tetramethylpiperidinol, 0.05mol (11.51g) of dimethyl sebacate and Add 100g of n-heptane into a 250mL three-necked flask with mechanical stirring, water separator and condenser, add ground 0.09mol (2.20g) lithium hydroxide and 0.003mol (1g) tetrabutylammonium bromide, and heat up To the reflux state (about 98°C), the methanol was continuously refluxed to separate, and the reaction was stopped when no methanol was separated after reflux (about 5h).
[0061] (2) Purification: After the reaction, cool down to room temperature, add 40mL×3 deionized water to wash 3 times, distill off the solvent and unreacted 1-methoxy-2,2,6,6-tetramethyl under reduced pressure Piperidinol obtained 27.47g of crude product, added the crude product into 55g m (methanol): m (water) = 0.8:1 mixed solvent, stirred for ...
Embodiment 2
[0070] A preparation method of bis(1-ethoxy-2,2,6,6-tetramethylpiperidin-4-yl) sebacate, comprising the steps of:
[0071] (1) Synthesis: 0.11mol (22.11g) of 1-ethoxy-4-hydroxyl-2,2,6,6-tetramethylpiperidinol, 0.05mol (11.51g) of dimethyl sebacate and Add 100g of n-heptane into a 250mL three-necked flask equipped with a mechanical stirrer, a water separator, and a condenser, and add 0.09mol (2.20g) of lithium hydroxide and 0.003mol (about 1g) of tetrabutylammonium bromide after grinding, Raise the temperature to the reflux state (about 98°C), continuously reflux to separate methanol, and stop the reaction when no methanol is separated after reflux (about 5h).
[0072] (2) Purification: After the reaction, cool down to room temperature, add 40mL×3 deionized water to wash, and distill off the solvent and unreacted 1-ethoxy-2,2,6,6-tetramethylpiperidine under reduced pressure Alcohol yielded 26.95g of the crude product. Add the crude product to 55g of m (methanol): m (water) = 0...
Embodiment 3
[0080] A preparation method of bis(1-propoxy-2,2,6,6-tetramethylpiperidin-4-yl) sebacate, comprising the steps of:
[0081] (1) Synthesis: 0.11mol (23.65g) of 1-propoxy-4-hydroxyl-2,2,6,6-tetramethylpiperidinol, 0.05mol (11.51g) of dimethyl sebacate and 100g of n-heptane was added to a 250mL three-necked flask equipped with a mechanical stirrer, a water separator and a condenser, and 0.09mol (2.20g) of lithium hydroxide and 0.003mol (about 1g) of tetrabutylammonium bromide were added into the ground, Raise the temperature to the reflux state (about 98°C), continuously reflux to separate methanol, and stop the reaction when no methanol is separated after reflux (about 5h).
[0082] (2) Purification: After the reaction, cool down to room temperature, add 40mL×3 deionized water to wash, and distill off the solvent and unreacted 1-propoxy-2,2,6,6-tetramethylpiperidine under reduced pressure The crude product was 30.65g, and the crude product was added to 60g m (methanol): m (wate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com