Preparation method of 4-amino-3-fluorobenzoic acid
A technology of fluorobenzoic acid and aminobenzonitrile, which is applied in the field of compound preparation, can solve the problems of high raw material prices, large environmental pollution, and large safety hazards, and achieve the effects of low cost, small environmental pollution, and easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] In a preferred embodiment, the present invention provides a kind of preparation method of 4-amino-3-fluorobenzoic acid, and synthetic route is as shown in following reaction formula, mainly comprises the following steps:
[0050]
[0051] (1) Ammonification: react 3,4-difluorobenzonitrile and ammonia in an autoclave to obtain the intermediate 3-fluoro-4-aminobenzonitrile;
[0052] (2) Hydrolysis: The intermediate 3-fluoro-4-aminobenzonitrile obtained in step (1) undergoes a hydrolysis reaction under alkaline conditions to generate 4-amino-3-fluorobenzoic acid.
[0053] Preferably, the amination reaction described in step (1) is: first add 3,4-difluorobenzonitrile and solvent (or no solvent) into the autoclave, then feed ammonia gas, and heat up to 60-150°C Reaction, take the reaction material liquid chromatography (LC) and control the reaction to complete. When the solvent exists, reduce the temperature to 10-30°C and release the pressure. According to the type of s...
Embodiment 1
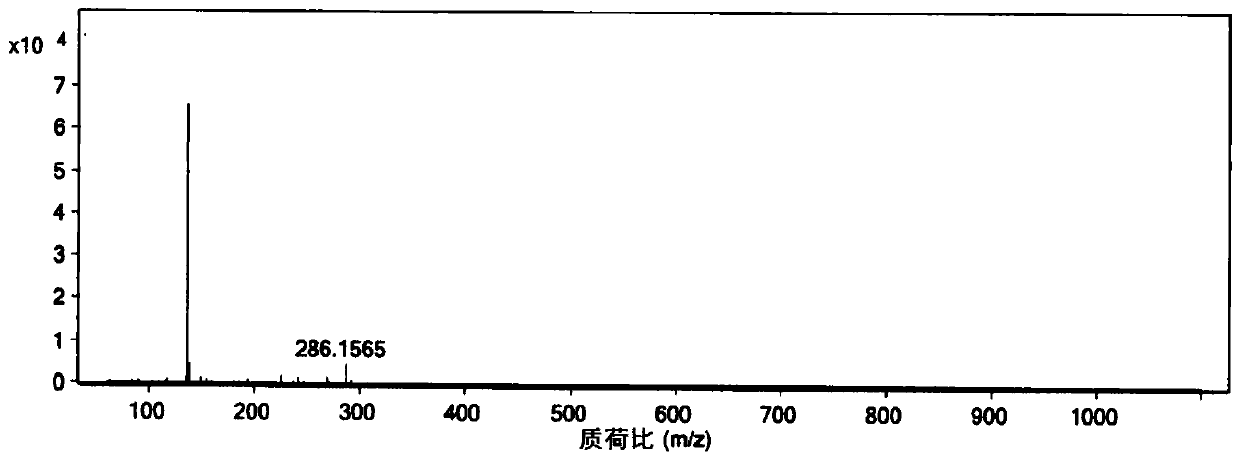
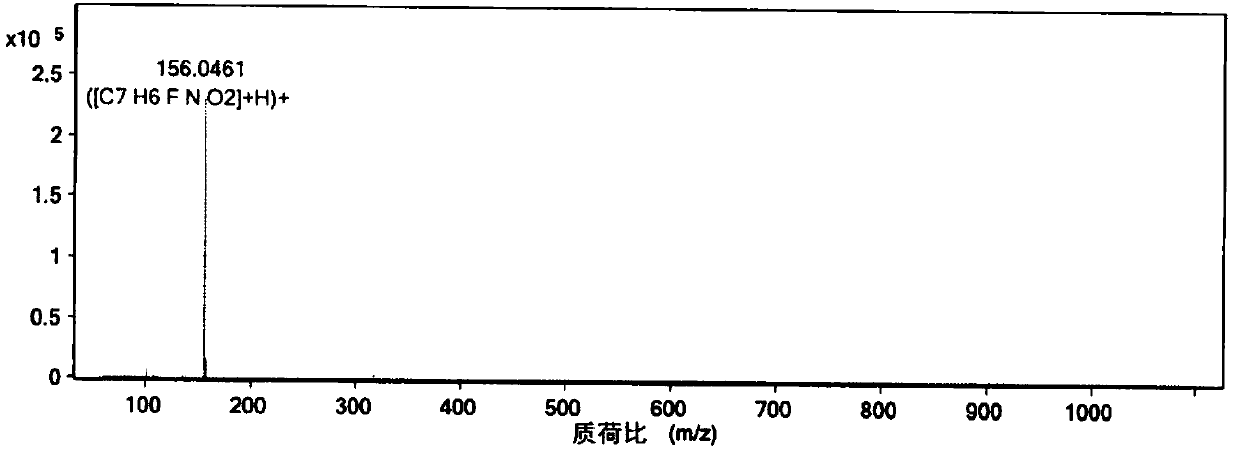
[0073] Step 1: Add 3,4-difluorobenzonitrile (30 g, 0.22 mol) into a 500 mL autoclave, and feed 7.5 g (0.44 mol) of ammonia gas. Then the temperature was raised to 90° C. to react for 24 hours (the maximum pressure during the reaction was 4.0 Mpa), and samples were taken, and the reaction was monitored by liquid chromatography until the normalized content of raw materials was ≤0.5%. After cooling down to room temperature and releasing the pressure, add methyl tert-butyl ether (90mL) to dissolve the material; wash with water (150mL×3) to remove the ammonium fluoride generated by the reaction, separate the organic phase, and concentrate under reduced pressure until it does not flow to obtain a crude product . Recrystallize with toluene (120mL), cool down to 0°C and filter, rinse the filter cake with cold toluene (0-5°C) once, and dry under vacuum at 40°C to obtain 8.8g of 3-fluoro-4-aminobenzonitrile (yield: 30%; purity>99%; off-white solid, melting point 70-72°C).
[0074] Whe...
Embodiment 2
[0088] Step 1: Add 3,4-difluorobenzonitrile (30g, 0.22mol) into a 500mL autoclave, and feed 15g (0.88mol) of ammonia gas. Then the temperature was raised to 90° C. to react for 24 hours (the maximum pressure during the reaction was 4.0 Mpa), and samples were taken, and the reaction was monitored by liquid chromatography until the normalized content of raw materials was ≤0.5%. After cooling down to room temperature and releasing the pressure, add ethyl acetate (90 mL) to dissolve the material; wash with water (150 mL x 3) to remove the ammonium fluoride generated by the reaction, separate the organic phase, and concentrate under reduced pressure until it stops flowing to obtain a crude product. Recrystallize with toluene (120mL), cool down to 0°C and filter, rinse the filter cake once with cold toluene (0-5°C), and dry under vacuum at 40°C to obtain 27.9g of 3-fluoro-4-aminobenzonitrile (yield: 95%; purity>99%; off-white solid, melting point 70-72°C), the intermediate was detec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com