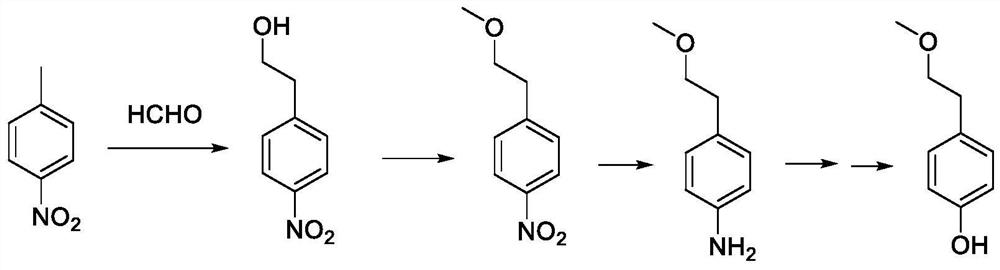
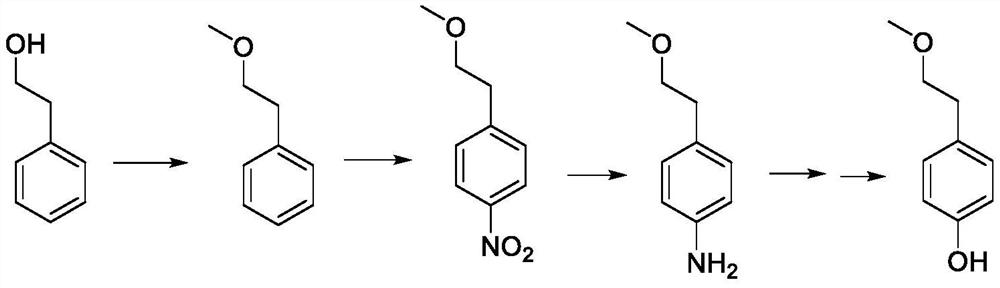
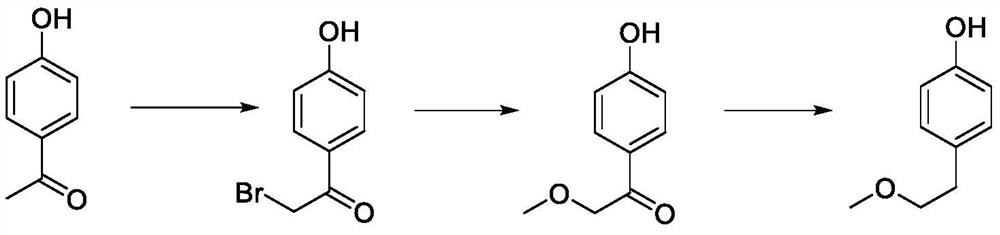
A kind of preparation method of p-(2-methoxy)ethylphenol
A technology of ethyl phenol and chloroethyl phenol, which is applied in the field of organic synthetic medicinal chemistry, can solve problems such as difficult access to raw materials, difficulty in industrialization, and difficulty in post-processing, and achieve the effects of improved convenience, easy reaction, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058]
[0059] Add 115g of p-chlorophenol, 260g of toluene, 3g of concentrated sulfuric acid into a 1L four-neck flask, stir evenly, and slowly introduce 120g of isobutylene at an internal temperature lower than -10°C. Quenched with % NaOH, washed again with 60 g of 30% NaOH, washed once with 60 g of water, removed toluene, and concentrated to obtain intermediate I-Cl (150 g, purity 99.2%, yield 90%).
Embodiment 2
[0061]
[0062] Add 150g of p-bromophenol, 350g of toluene, 5g of concentrated sulfuric acid into a 1L four-neck flask, stir evenly, and slowly feed 130g of isobutylene at an internal temperature lower than -10°C for 4 hours, keep at -10°C-0°C for 12h, add 120g of 30 Quenched by % NaOH, washed again with 60g of 30% NaOH, washed once with 60g of water, toluene was removed, and the product was concentrated to obtain intermediate I-Br (161g, purity 98.3%, yield 81%).
Embodiment 3
[0064]
[0065] Add 23g magnesium chips, 150g THF, N 2 Protection, heating at 65°C, dissolving 150g of intermediate I-Cl obtained in Example 1 in 150g THF and adding slowly, keeping the reaction at 72°C for 16h, cooling the reaction solution to 0-10°C, slowly adding 43g of ethylene oxide dropwise, After the addition, keep warm at 10°C for 2 to 3 hours, add 300g of 30% sulfuric acid solution dropwise, extract with 150g of toluene, wash the organic phase with 100g of water once, dry, recover toluene and THF, and obtain 150g of intermediate II crude product with a purity of 91%. 88%. The crude product can be directly subjected to the next reaction or purified by vacuum distillation to obtain 120 g of pure product with a purity of 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com