Fluorescent probe detecting cyanide ions and hypochlorous acid and preparation and application thereof
A technology of cyanide ions and probes, applied in the field of fluorescent probes for detecting cyanide ions and hypochlorous acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
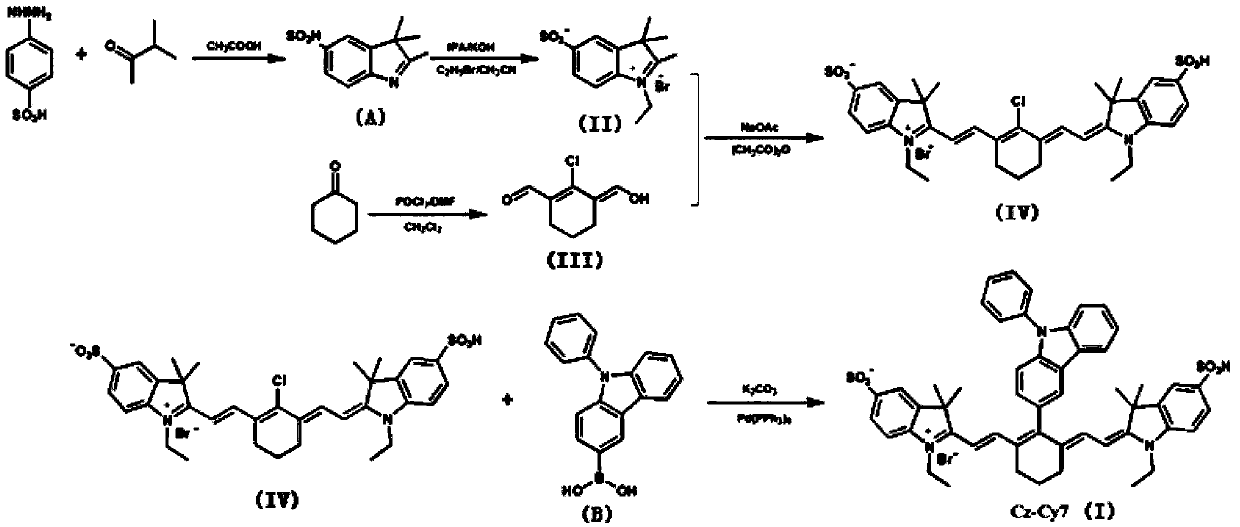
[0094] Embodiment 1: the synthesis of target probe Cz-Cy7
[0095] (1) Synthesis of potassium N-ethyl-2,3,3-trimethylindole-5-sulfonate (compound (II))
[0096] Into a 50mL single-necked bottle, add 10mL of acetic acid, phenylhydrazine-4-sulfonic acid (2.000g, 0.010mol) and methyl isopropyl ketone 4.00mL (0.037mol) in sequence, and reflux for 8 hours under nitrogen protection. At this time, the solution Turned dark red. After cooling to room temperature, the reaction solution was slowly dropped into ethyl acetate, and a pink solid precipitated out of the solution; suction filtered, washed several times with ether, and dried in vacuo; the above-mentioned dried pink powder was dissolved in an appropriate amount of methanol, and Dissolve 0.561g of potassium hydroxide in isopropanol to make a saturated solution, then add dropwise a saturated solution of potassium hydroxide in isopropanol to the methanol solution to obtain a yellow solid, which is centrifuged, washed 3-4 times wit...
Embodiment 2
[0104] Embodiment 2: the titration experiment of cyanide ion pair probe
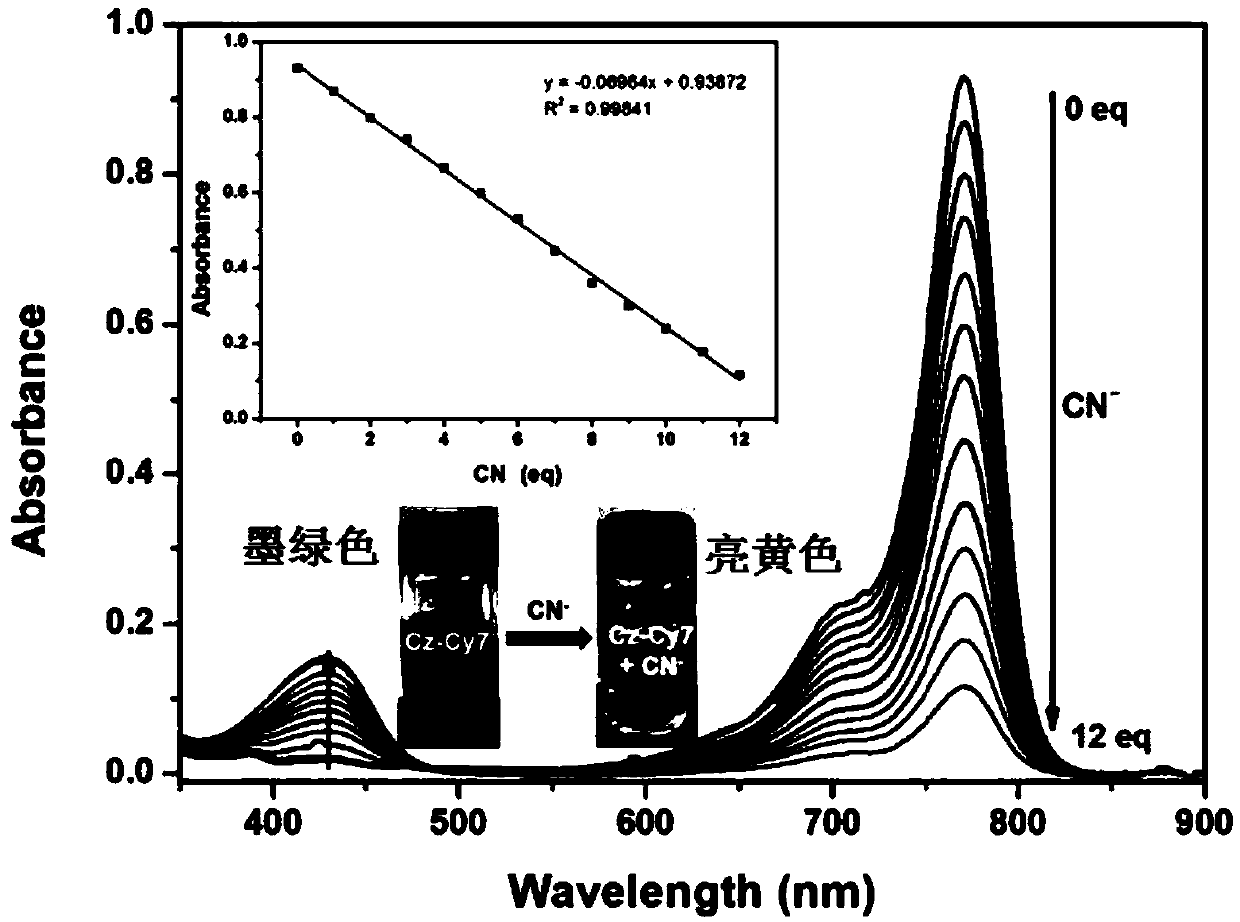
[0105] In a mixed solvent of water and DMF (volume ratio 3:7, preferably adding HEPES buffer solution, pH=10), prepare a probe solution with a concentration of 5 μM. Then drop different concentrations of cyanide ions (0μM, 5μM, 10μM, 15μM, 20μM, 25μM, 30μM, 35μM, 40μM, 45μM, 50μM, 55μM, 60μM), after 5min, through the ultraviolet-visible absorption spectrum and fluorescence spectrum test Absorption and fluorescence emission responses of mixed systems.
[0106] Depend on image 3 It can be seen that the intensity of the absorption peak of the probe at 771 nm decreases with the increase of the concentration of cyanide ions, and the color of the solution changes from dark green to bright yellow.
[0107] Depend on Figure 4 It can be seen that the emission peak intensity of the probe at 806 nm decreases with the increase of the cyanide ion concentration, the probe reacts completely with 12 equivalents of ...
Embodiment 3
[0108] Embodiment 3: The reaction kinetics test of probe to cyanide ion
[0109] At room temperature, prepare a probe solution with a concentration of 5 μM (water / DMF, a volume ratio of 3:7, preferably adding a HEPES buffer solution, pH=10), then add cyanide ions with a microsyringe and record the sample at 771 nm at different times UV absorption intensity at .
[0110] Depend on Figure 5 It can be seen that as time increases, the absorption intensity of the probe at 771nm decreases, and after 5 minutes, the ultraviolet absorption intensity of the system does not change further with time, indicating that the probe can complete the reaction to cyanide within 5 minutes. ion detection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com