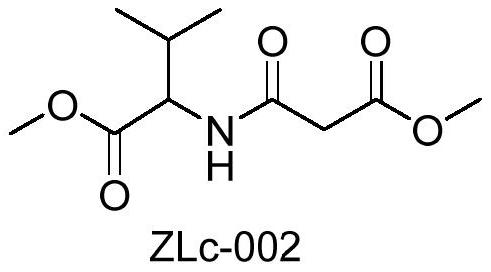
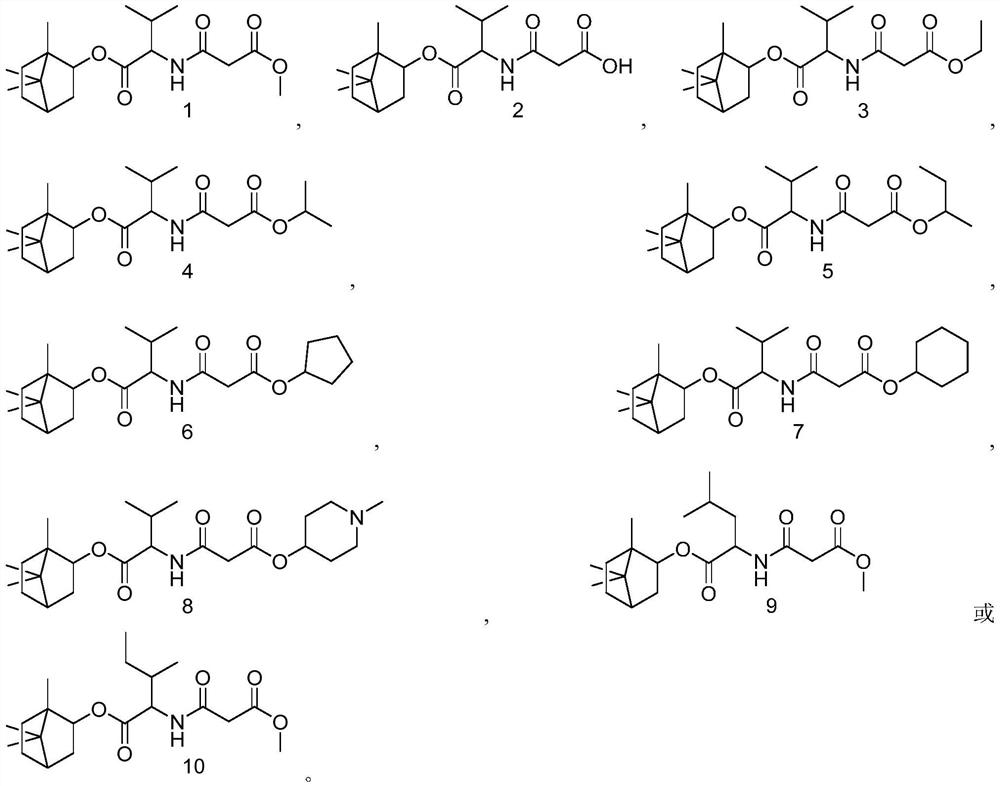
A class of derivatives of malonic acid monoester acylated amino acid (+) 2-alcohol esters and their applications
A technology for the acylation of malonate monoester and amino acids, which is applied to the active ingredients of heterocyclic compounds, medical preparations containing active ingredients, and drug combinations, etc., and can solve problems such as failure to obtain effects, respiratory failure, and poor drug efficacy , to achieve the effect of reducing ischemic injury and excellent neuroprotective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] The following examples can be understood by those skilled in the art, but the present invention is not intended in any way. Example 1: Synthesis of Target Compound:
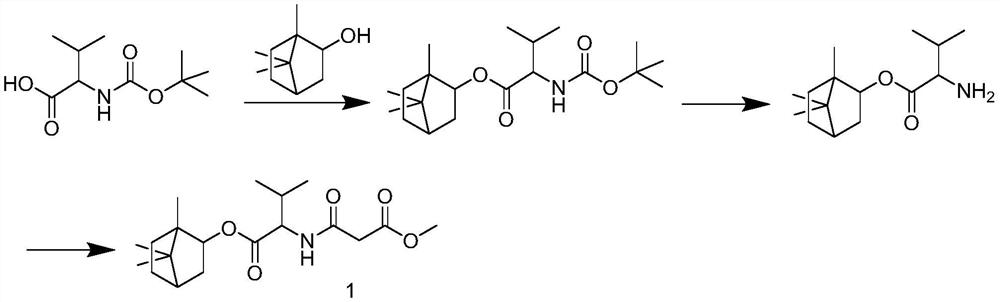
[0015] 1.1 Synthesis of Target Compound 1:
[0016] synthetic route:
[0017]
[0018] Operation: Boc-D-VAL (1.54 g, 10 mmol), (+) 2-崁 alcohol (3.26 g, 15 mmol) was added to 100 mL of the solution, and 50 ml of dichloromethane is dissolved, and then add EDCI to the solution. (3.6 g 20 mmol), and DMAP (2 mmol), the mixture was stirred at room temperature for about 6 h, TLC monitored the reaction process, which was developed with phosphomolybdic acid. Spun decompression, rapid column chromatography obtained 3.2 g of colorless oil (mobile phase: petroleum ether: ethyl acetate, volume ratio = 20: 1). Dissolved with 10 ml of ethyl acetate, a dry HCl gas was introduced, and the TLC was monitored after 15 min, and a large amount of white solid was halved under reduced pressure, slurry with diethyl ether and filtere...
Embodiment 2
[0037] Example 2: Target compounds on neuroprotective effects
[0038] 2.1 Cultivation of primary neurons
[0039] ICR mice with agents of 15 to 16 days were taken, and after the cervical spine was dislocated, the uterus was separated from the placenta, and the removed fetal rats were placed in a 0.1% new neutral solution, and 75% sterilized. The left hand fixed counter, the right hand is separated by the eye, revealing the brain hemisphere, cauting with the eyes of the two sides of the cerebral cortex, placed in a platter with 10 ml D-HANKS. After all were taken, the mening film was released in another 1 plate with 10 ml D-HANKS, and then 5 ml D-HANKS washed once. The cortex was cut into the plate with a 37 ° C incubator (2 ml 0.25% pancreatic enzyme + 2 ml D-Hanks), and transferred to the small beaker, mixed into the small beaker. Digestion in the box for 10 min. After taking out from the incubator, 5 ml of DMEM + 10% FBS was added to terminate digestion, and after mixing, trans...
Embodiment 3
[0047] Example 3: Protective effect of target compound on focal cerebral ischemia-reperfusion
[0048] 3.1 Preparation of focal cerebral ischemia reperfusion model
[0049] After 2% hydrated chloroform (0.2 ml / 10g, IP) anesthesia, the animal is fixed to the operating table, cutting the skin along the neck, bluntly separating the neck muscle tissue, exposes the right neck pressure ( CCA) and be careful; gently peel the accompanying vagus nerve, ligation and cut the neck of the neck (ECA) of the inner and superficial walking (ECA); in the nearby sides Branch artery - pterygium artery, separating it. The CCA is close to the center of surgery; the distal end of the knotted line is scratched by the eye, and the surgery uses the nylon line (8 / 0) from the mouse to the blender, inserted, inserted along the small mouth. 16-17mm (mouse) feels slight resistance, and the front end of the line has reached the anterior artery. At this point, the blood supply of the brain medium artery (MCA) i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com