Arctigenin amino acid ester derivatives, preparation method and use thereof
A technology of arctigenin and amino acids, which is applied in drug combinations, nervous system diseases, organic chemistry, etc., can solve problems such as low pass rate, high plasma protein binding rate, and low bioavailability of the blood-brain barrier, achieving reasonable design, Ease of synthesis, significant neuroprotective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
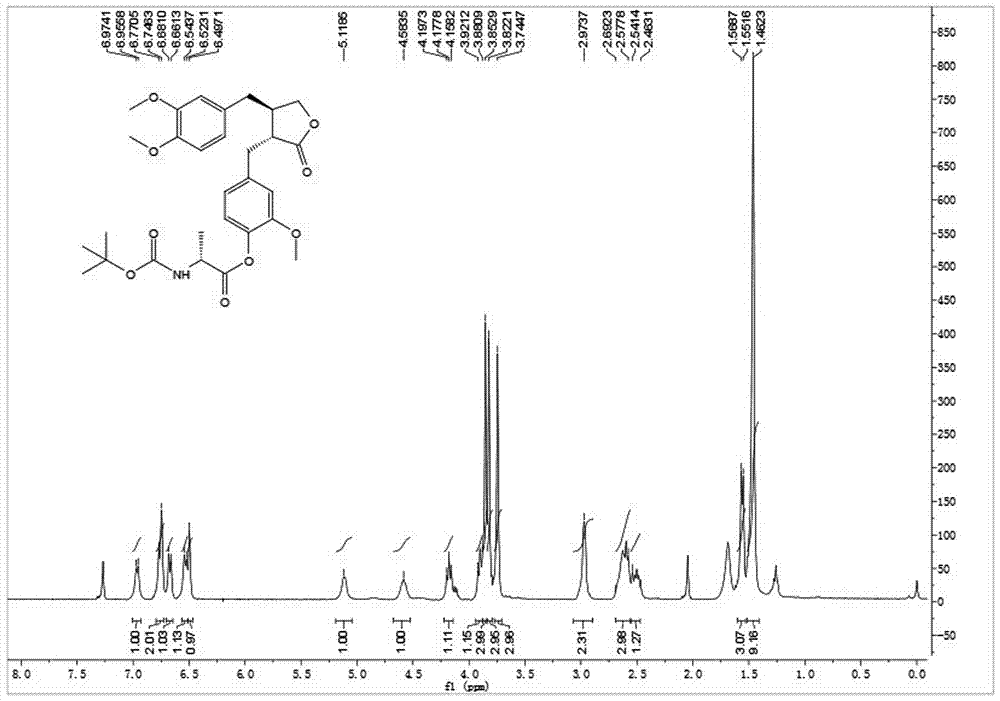
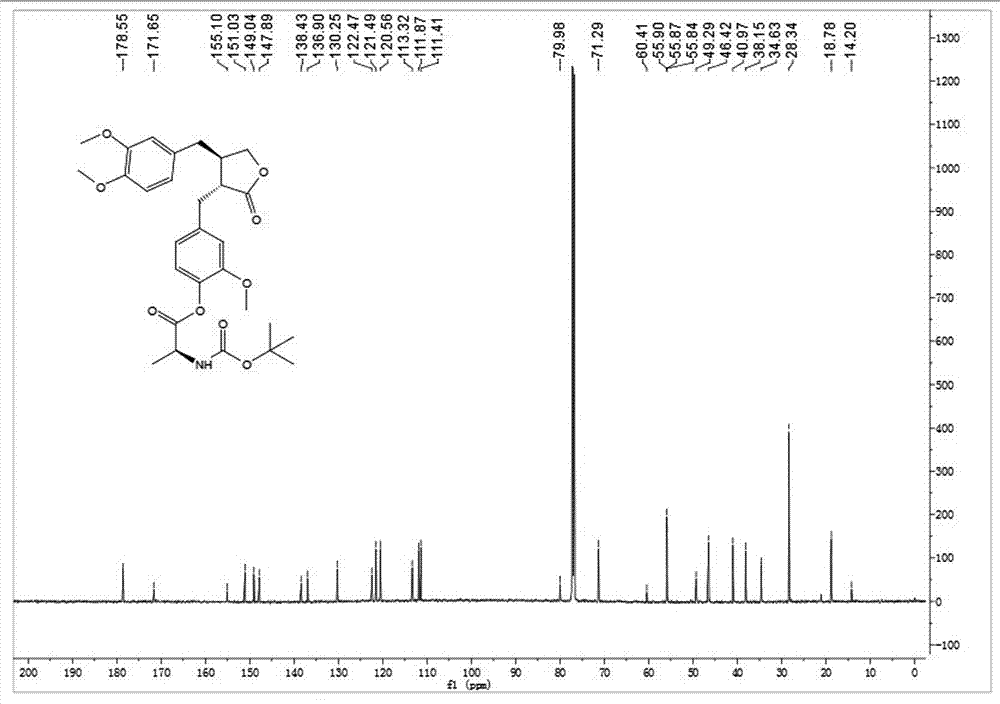
[0037] Embodiment 1: synthetic compound 1-1, please refer to figure 1 and figure 2
[0038] .
[0039] Will N -Boc- L-Alanine (30.4 mg, 0.16 mmol) was dissolved in dry toluene, 2,4,6-trichlorobenzoyl chloride (30.5 μL, 0.20 mmol) and DIPEA (26.0 μL, 0.20 mmol) were added, and after stirring for 10 min Arctigenin (50 mg, 0.134 mmol) was added, and DMAP (32.7 mg, 0.27 mmol) was added at one time, and stirring was continued at room temperature for 3 h. TLC showed that the reaction was almost complete. After concentration under reduced pressure, silica gel thin-layer preparation plate was purified to obtain 63 mg of white powder with a yield of 86%. [α] D 25 = -25.6 (c 1.0, CHCl 3 ); 1 H NMR (400 MHz, CDCl 3 ) δ 6.96 (d, J = 7.3 Hz, 1H), 6.76(d, J = 9.7 Hz, 2H), 6.67 (d, J = 7.9 Hz, 1H), 6.53 (d, J = 8.2 Hz, 1H), 6.50(s, 1H), 5.12 (m, 1H), 4.58 (m, 1H), 4.18 (t, J = 7.8 Hz, 1H), 3.93 – 3.88(m, 1H), 3.85 (s, 3H), 3.82 (s, 3H), 3.74 (s, 3H), 2.97 (s, 2H), 2.6...
Embodiment 2
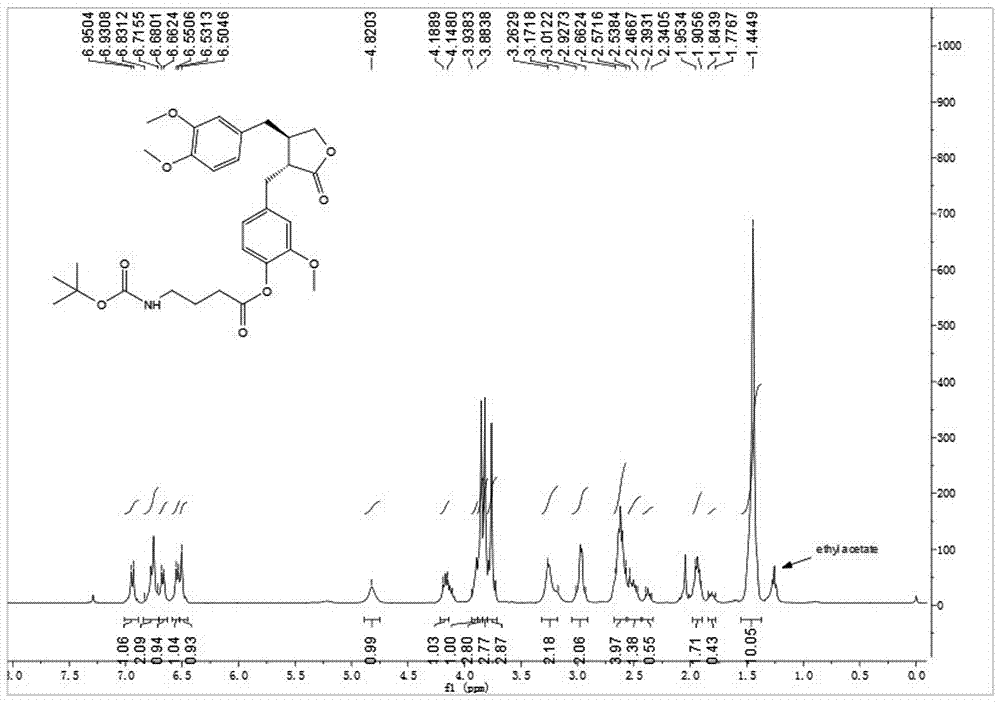
[0040] Embodiment 2: synthetic compound 1-2, please refer to image 3 and Figure 4
[0041] .
[0042] The CAS number is 57294-38-9 N -Boc-GAMMA-aminobutyric acid (32.5 mg, 0.16 mmol) was dissolved in dry toluene, 2,4,6-trichlorobenzoyl chloride (30.5 μL, 0.20 mmol) and DIPEA (26.0 μL, 0.20 mmol) were added, After stirring for 10 min, arctigenin (50 mg, 0.134 mmol) was added, and DMAP (32.7 mg, 0.27 mmol) was added at one time, and stirring was continued at room temperature for 3 h. TLC showed that the reaction was almost complete. After concentration under reduced pressure, it was purified by silica gel thin-layer preparative plate to obtain 65 mg of white powder with a yield of 87%. [α] D 20 =-9.5( c 1.0, CHCl 3 ). 1 H NMR (400 MHz, CDCl 3 ) δ 6.94 (d, J =7.9 Hz, 1H), 6.83 – 6.72 (m, 2H), 6.67 (d, J = 7.1 Hz, 1H), 6.54 (d, J = 7.7Hz, 1H), 6.50 (s, 1H), 4.82 (s, 1H), 4.19 – 4.15 (m, 1H), 3.93 – 3.88 (m,1H), 3.85 (s, 3H), 3.82 (s, 3H), 3.76 (s, 3H), 3.26 –...
Embodiment 3
[0043] Embodiment 3: synthetic compound 1-3, please refer to Figure 5 and Figure 6
[0044] .
[0045] Will N -Boc-glycine (28.0 mg, 0.16 mmol) was dissolved in dry toluene, 2,4,6-trichlorobenzoyl chloride (30.5 μL, 0.20 mmol) and DIPEA (26.0 μL, 0.20 mmol) were added and stirred for 10 min Arctigenin (50 mg, 0.134 mmol) was added, and DMAP (32.7 mg, 0.27 mmol) was added at one time, and stirring was continued at room temperature for 3 h. TLC showed that the reaction was almost complete. After concentration under reduced pressure, it was purified by silica gel thin layer preparative plate to obtain 59 mg of white powder with a yield of 83%. [α] D 20 =-16.3( c 1.0, CHCl 3 ), 1 H NMR (400 MHz, CDCl 3 , mixture of rotamers) δ 6.95 (d, J = 8.0Hz, 0.7H), 6.81 (d, J = 7.9 Hz, 0.3H), 6.76 – 6.72 (m, 1.7H), 6.67 – 6.62 (m,1H), 6.61 – 6.59(m, 0.3H), 6.54 – 6.51 (m, 1H), 6.49 – 6.45 ( m, 1H), 5.64(br, 0.3H), 5.10 (br, 0.7H), 4.21 – 4.15 (m, 2H), 4.15 – 4.07 (m, 1H), 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com