Multiple PCR detection kit for pathogens of respiratory tract, application and application method thereof
A technology for detection kits and respiratory tracts, applied in the field of nucleic acid amplification, can solve the problems of valuable information for patients, obstacles to large-scale use, and limited detection items, and achieve the effects of shortening performance, shortening detection time, and saving time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0118] Determination of solution components in the nucleic acid extraction reagent of embodiment 2
[0119] 1. Reaction system
[0120] 1) PCR reaction solution
[0121] 10×Buffer for Taq 15%; MgCl 2 dATP 0.2 mM; dCTP 0.2 mM; dGTP 0.2 mM; dUTP 0.4 mM; BSA 1.5 mg / mL and glycerol 5%.
[0122] 2) PCR enzyme solution
[0123] Taq DNA Polymerase 15%; UNG 5%; Superscript V 4%; DTT 2mM; Glycerol 40%; Anti-Taq 15%.
[0124] 3) primer pair (same as in Example 1)
[0125] 4) Probe (same as in Example 1)
[0126] 5) Nucleic acid extraction product: using the nucleic acid extraction reagent of the present invention, 200 μL of clinical samples (nasopharyngeal swab or lung lavage fluid) were loaded, and 65 μL of elution buffer was eluted as the nucleic acid extraction product.
[0127] 2. Nucleic acid extraction reagents
[0128] Nucleic acid extraction reagent 1
[0129] Wherein the lysis binding solution includes the following components: 1% fatty acid methyl ester ethoxylate sod...
Embodiment 3
[0142] Example 3 Performance Verification of Detection Kit Primer Probes in Single and Multiplex PCR Systems
[0143] 1. Reaction system
[0144] 1) PCR reaction solution
[0145] 10×Buffer for Taq 15%; MgCl 2 dATP 0.2 mM; dCTP 0.2 mM; dGTP 0.2 mM; dUTP 0.4 mM; BSA 1.5 mg / mL and glycerol 5%.
[0146] 2) PCR enzyme solution
[0147] Taq DNA Polymerase 15%; UNG 5%; Superscript V 4%; DTT 2mM; Glycerol 40%; Anti-Taq 15%.
[0148] 3) primer pair: consistent with that in Example 1
[0149] 4) probe: consistent with that in Example 1
[0150] 5) Nucleic acid extraction product: consistent with that in Example 1
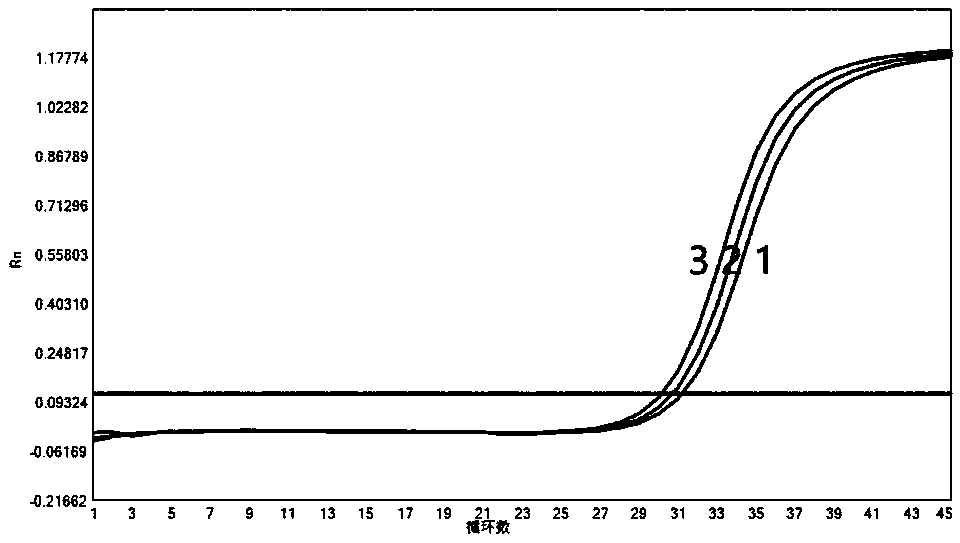
[0151] 2. Experimental process: select clinical samples of influenza A, influenza B, respiratory syncytial virus, parainfluenza 1, parainfluenza II, parainfluenza III, adenovirus, metapneumovirus, enterovirus or rhinovirus, for the detection of influenza A Influenza virus, the national reference product S1 is diluted E1, E2, E3, E4, E5, E6 times, the corresponding co...
Embodiment 4
[0157] Embodiment 4 detection kit specific detection
[0158] 1. Reaction system
[0159] 1) PCR reaction solution
[0160] 10×Buffer for Taq 15%; MgCl 2 dATP 0.2 mM; dCTP 0.2 mM; dGTP 0.2 mM; dUTP 0.4 mM; BSA 1.5 mg / mL and glycerol 5%.
[0161] 2) PCR enzyme solution
[0162] Taq DNA Polymerase 15%; UNG 5%; Superscript V 4%; DTT 2mM; Glycerol 40%; Anti-Taq 15%.
[0163] 3) primer pair: consistent with that in Example 1
[0164] 4) probe: consistent with that in Example 1
[0165] 5) Nucleic acid extraction product: consistent with that in Example 1
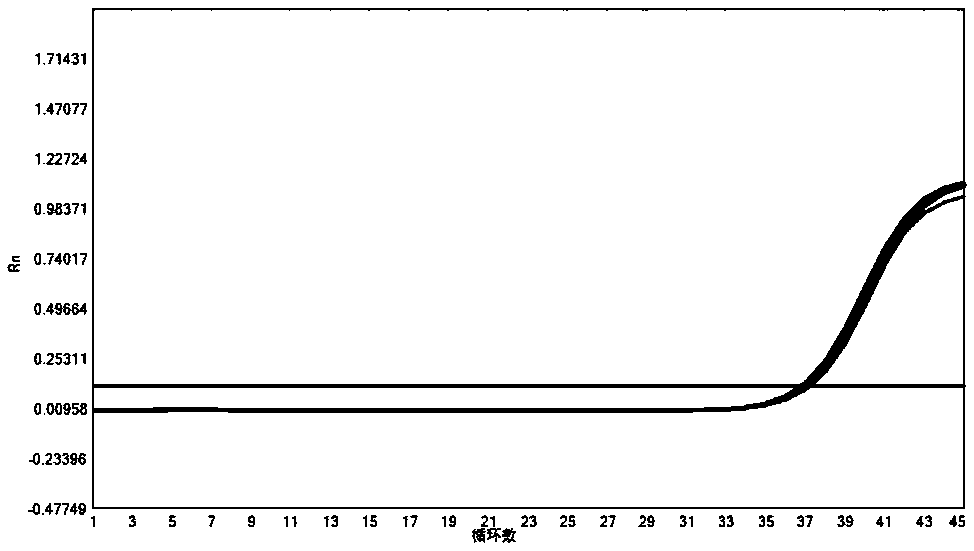
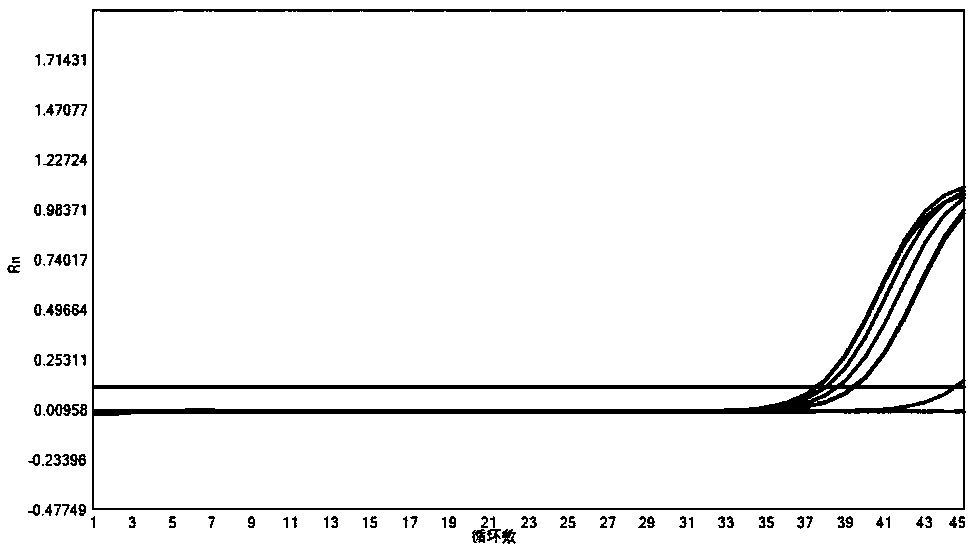
[0166] 2. Experimental process: Select clinical samples of other common respiratory pathogens, including Mycoplasma pneumoniae, Streptococcus pneumoniae, Haemophilus influenzae and human genome, for nucleic acid extraction, and compare the experimental results of each item in the multiplex PCR system. In the process of nucleic acid extraction, add internal quality control plasmid amplicon sequence SEQ ID NO:31, and add in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com