AST (astaxanthin)-supported phospholipid nanoparticles as well as preparation method and application thereof
A penicillin phospholipid and nanoparticle technology, which is used in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve unfavorable medicines, increase the probability of human trauma and infection, To reduce the frequency of administration, improve biocompatibility, and improve water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Preparation of astaxanthin phospholipid nanoparticles (AST-LPN) by nanoprecipitation method
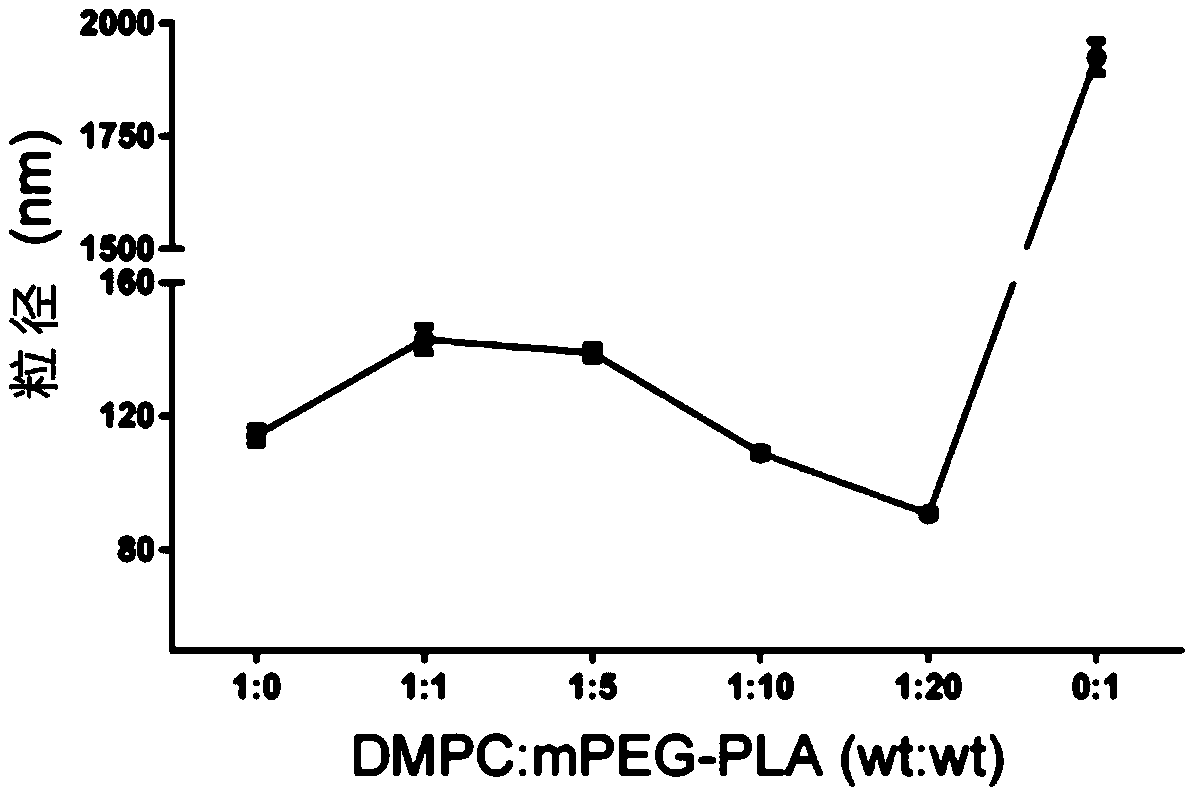
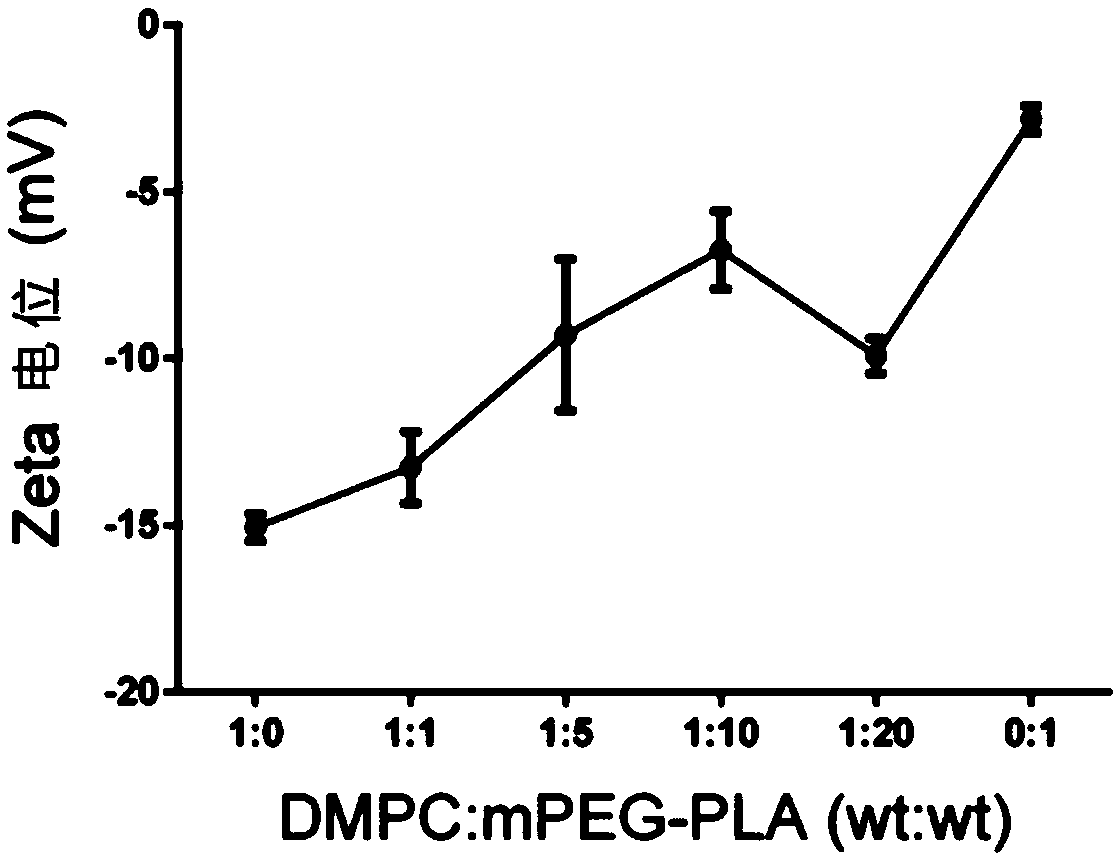
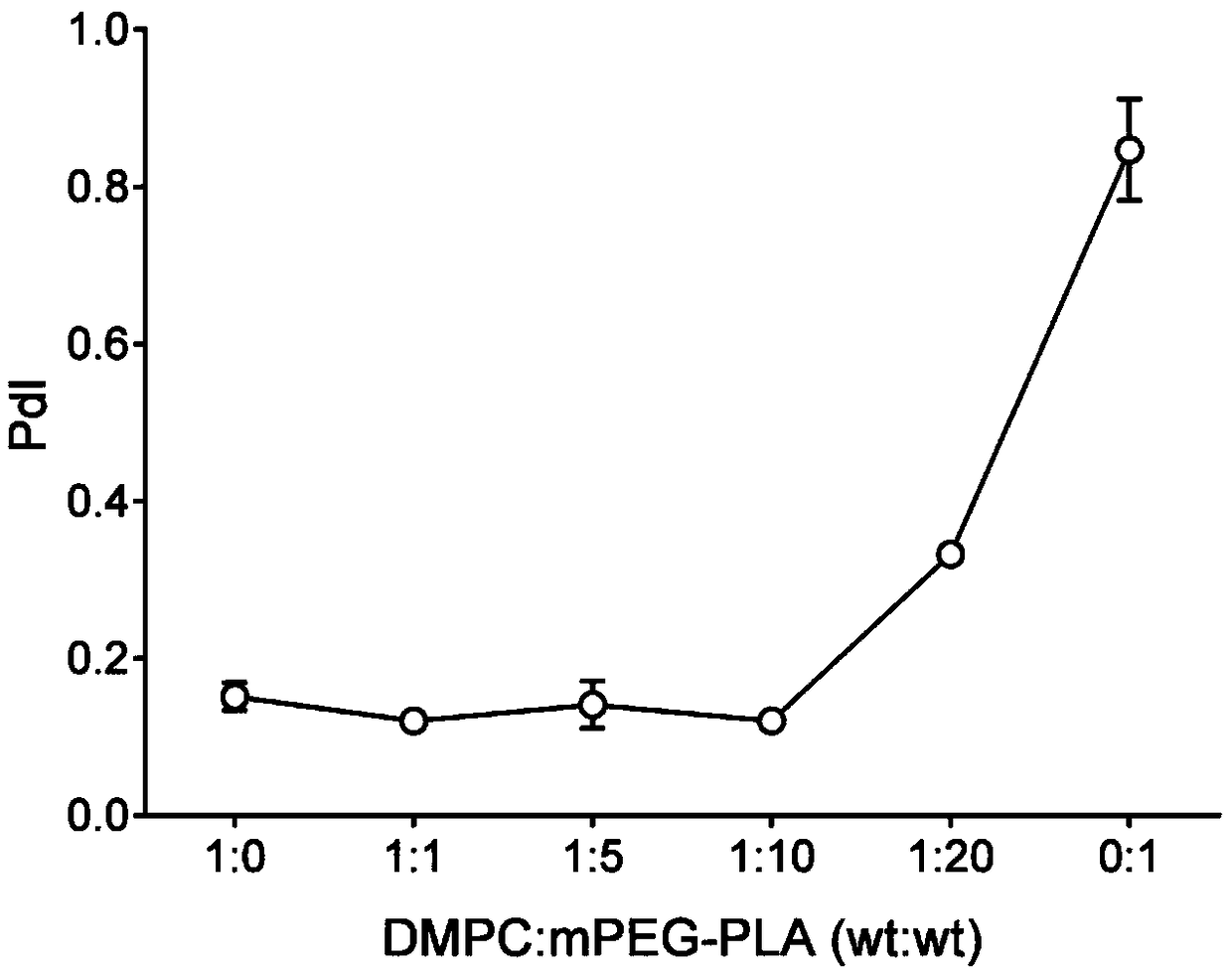
[0052] 30mg MPEG-PLA (polymer) and 2-5mg AST were dissolved in 1mL acetonitrile solution as the organic phase; 3mg DMPC (phospholipid) was dissolved in 10mL 4% ethanol solution as the water phase. The water phase was preheated to 65°C and stirred at 900 rpm until DMPC was completely dissolved. The organic phase containing the drug was added dropwise, and the dripping was completed within 3 minutes, and the stirring was continued for 2 minutes, the stirring speed was adjusted to 300 rpm, and the organic phase was stirred gently overnight until the organic phase was completely volatilized. Use a Millipore 10kDa centrifugal concentration tube to centrifuge at 4000g, 4°C for 40min to remove unencapsulated drugs and organic solvents, and centrifuge twice continuously to obtain about 500μL nanoparticle concentrate. The mass ratio of DMPC to MPEG-PLA is 1:0, 1:1, 1:5, 1:10...
Embodiment 2
[0057] Embodiment 2 Emulsion solvent volatilization method prepares astaxanthin phospholipid nanoparticles (AST-LPN)
[0058] Analysis of the main reasons for the low encapsulation efficiency and drug loading of astaxanthin lipid nanoparticles prepared by nanoprecipitation method may be due to the low solubility of AST in acetonitrile. The same mass of AST was dissolved in acetonitrile and dichloromethane. After vortex dissolving, the dichloromethane solution became clearer and brighter. It is speculated that the solubility of AST in dichloromethane is higher than that of acetonitrile solution. Therefore, dichloromethane was used instead of acetonitrile to dissolve AST and MPEG-PLA, and 4% (volume ratio of methanol to water) methanol aqueous solution was used to dissolve DMPC to prepare AST-LPN. The detailed steps are as follows: 30mg MPEG-PLA and 2-5mg AST were dissolved in 1mL dichloromethane as the organic phase; 3mg DMPC was dissolved in 3mL 4% aqueous methanol as the aque...
Embodiment 3
[0061] Example 3 Investigation of the sustained release characteristics of AST-LPN in inner ear lymph fluid
[0062] Such as Figure 9 As shown, AST-LPN was sustained release in artificial lymph fluid for 15 days in vitro. In addition, if Figure 10 As shown, the experimental results of drug concentration detection in inner ear lymph fluid proved that after AST solution (6 μg) was administered through the round window membrane, the lymph fluid was taken 30 minutes after administration for mass spectrometry analysis, and the AST content was not detected. The results suggest that fat-soluble AST cannot enter the inner ear through the round window membrane. Based on this, we determined that AST administered through the round window membrane could not exert the protective effect of cisplatin ototoxicity. see Figure 10 , after guinea pigs were given AST-LPN through the round window membrane (RWM) (AST concentration: 1.0mg / mL, 6μL), the concentration in the lymph fluid reached ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com