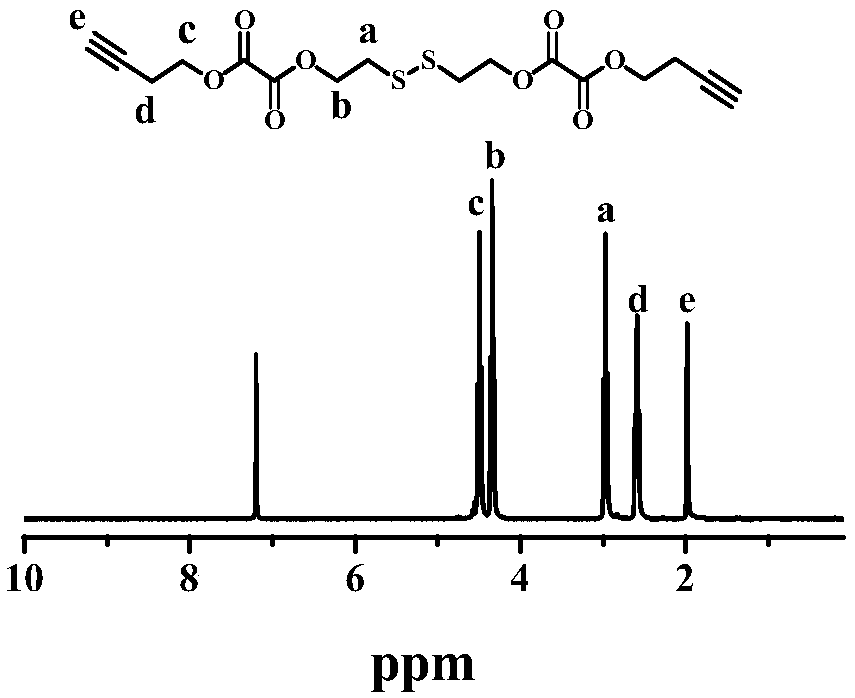
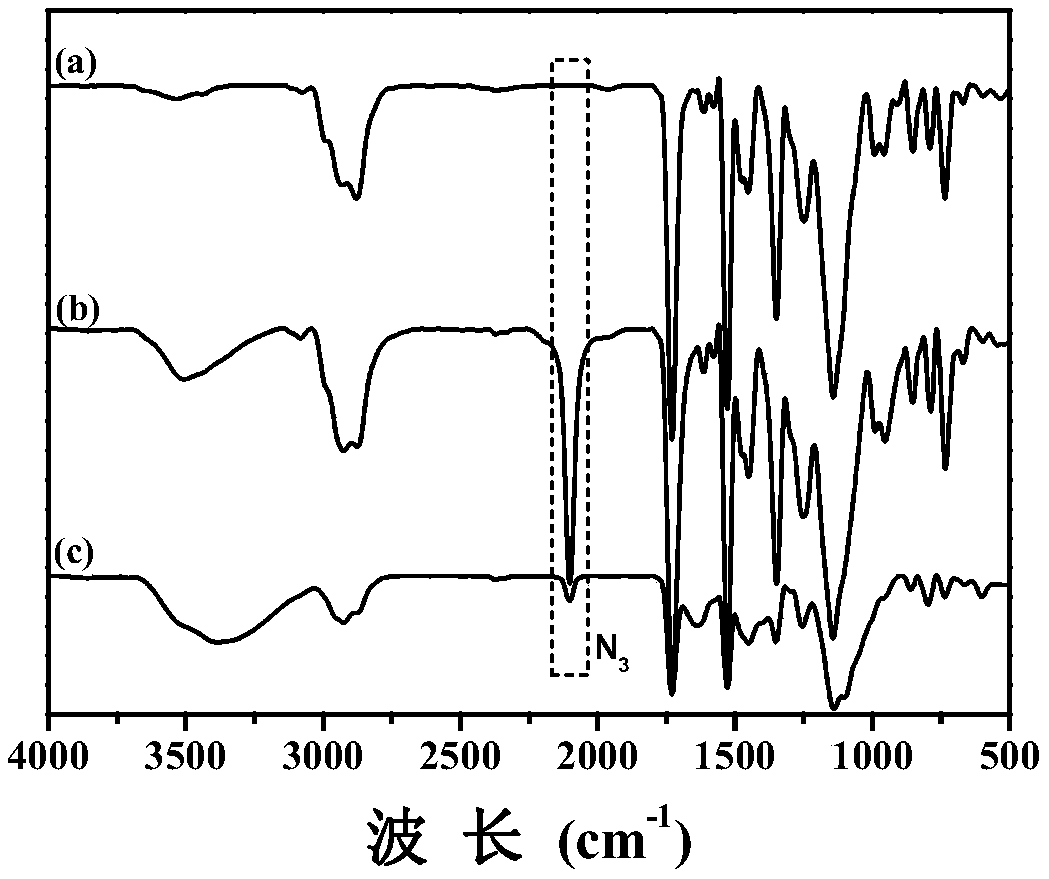
Triple stimulation responsive core cross-linked polymeric micelle as well as preparation method and application thereof
A cross-linked polymer and stimuli-responsive technology, which is applied in drug delivery, drug combination, pharmaceutical formulation, etc., can solve the problems of few research reports, and achieve the effect of simple operation, mild reaction conditions, and good control release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046]
[0047]
[0048] 1. Dissolve mPEG-Br (0.2g, 0.1mmol), NBM (1.55g, 7mmol), GMA (0.426g, 3mmol), PMDETA (0.0173g, 0.1mmol) in 4mL DMF, freeze-thaw and degas continuously 2 Add CuBr (0.0143g, 0.1mmol) once more, and then freeze-thaw and degas once more, and then react at 70°C for 24 hours under a nitrogen atmosphere. After the reaction, dilute with THF, pass through a neutral alumina column, and rotate to remove After most of the solvent, dialyze in deionized water for 3 days using a dialysis bag with a molecular weight cut-off of 8 kDa, and freeze-dry to obtain a white solid, which is the amphiphilic diblock polymer shown in formula I-1.
[0049] 2. The amphiphilic diblock polymer (0.4g, 0.86mmol) shown in formula I-1 and NaN 3 (0.25g, 3.87mmol), NH 4 Cl (0.21g, 3.87mmol) was dissolved in 20mL DMF, and reacted at 50°C for 24 hours under stirring. After the reaction, the insoluble salt was filtered off with a Buchner funnel. The bag was dialyzed in deionized water...
Embodiment 2
[0054]
[0055] 1. Dissolve mPEG-Br (0.4g, 0.2mmol), NBM (2.21g, 10mmol), GMA (1.42g, 10mmol), PMDETA (0.0346g, 0.2mmol) in 4mL DMF, freeze-thaw and degas continuously 2 Add CuBr (0.0286g, 0.2mmol) once more, and then freeze-thaw degassing once more, and then react at 70°C for 24 hours under a nitrogen atmosphere. After the reaction, dilute with THF, pass through a neutral alumina column, and rotate to remove After most of the solvent, use a dialysis bag with a molecular weight cut-off of 8KDa to dialyze in deionized water for 3 days, and freeze-dry to obtain a white solid, which is the amphiphilic diblock polymer shown in formula I-2.
[0056] 2. The amphiphilic diblock polymer (0.4g, 1.05mmol) shown in formula I-2 and NaN 3 (0.34g, 5.25mmol), NH 4 Cl (0.28g, 5.25mmol) was dissolved in 20mL DMF, and then reacted at 50°C for 24 hours under stirring. After the reaction, the insoluble salt was filtered off with a Buchner funnel. The bag was dialyzed in deionized water for 3...
Embodiment 3
[0061] Application of the triple stimulus-responsive nuclear cross-linked polymer micelles prepared in Example 1 as hydrophobic drug carriers
[0062] The azide-modified amphiphilic diblock polymer (30 mg, 0.06 mmol) shown in formula II-1, Nile red (1.5 mg, 0.005) and the cross-linking agent (17 mg, 0.04 mg) shown in formula III-1 mmol) was dissolved in 20mL THF, micelles were formed by dialysis, and then CuSO was added 4 ·5H 2 O (30mg, 0.12mmol) and sodium ascorbate (24mg, 0.12mmol), reacted at 25°C for 48 hours, and then dialyzed the unreacted cross-linking agent with an 8kDa dialysis bag to obtain triple stimulus responsiveness to light, oxidation and reduction. Core-crosslinked polymer drug-loaded micelles.
[0063] Fluorescence spectrometer detection (excitation wavelength is 560nm, micelle concentration is 0.2mg / mL) release curve of triple stimulus-responsive nuclear cross-linked polymer drug-loaded micelles under single stimulation and multiple stimulation, the result...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com