Oral solid preparation for treating hypertension and related diseases
A technology for solid preparations and high blood pressure, which is applied in cardiovascular system diseases, metabolic diseases, urinary system diseases, etc., and can solve problems such as viscous active substances, difficult preparations, and poor fluidity of prescriptions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Embodiment 1: Preparation of QR01019K
[0084] Dissolve QR01019 (1.0g) in dichloromethane (5ml), stir at room temperature to form a solution, add potassium phthalimide (0.27g) to the solution, keep it warm for 4 hours, cool to -50°C, filter, The solid obtained by spin-drying the solvent is amorphous QR01019K.
[0085] Melting point: 135-145°C.
[0086] MS / HRMS m / z: 717[M+H] + ;677[M-K] - .
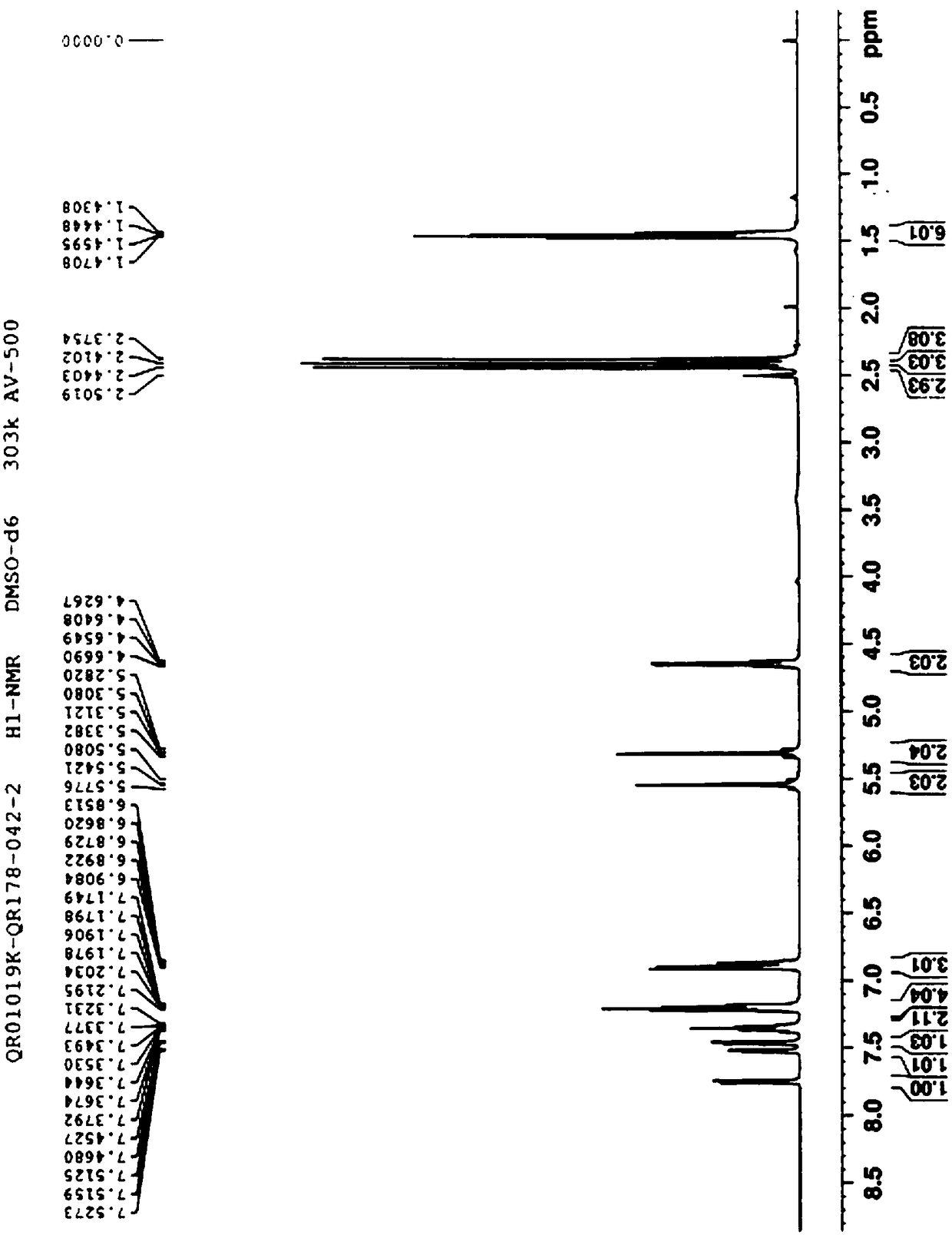
[0087] 1 H-NMR (400MHz, DMSO-d 6 )δ: 1.44(t, 3H), 1.46(t, 3H), 2.38(s, 3H), 2.41(s, 3H), 2.44(s, 3H), 4.64(q, 2H), 5.29(d, 1H ), 5.32(d, 1H), 5.52(d, 1H), 5.56(d, 1H), 6.86(q, 1H), 6.90(d, 2H), 7.18(m, 2H), 7.22(d, 2H) , 7.33 (m, 1H), 7.36 (m, 1H), 7.46 (d, 1H), 7.52 (dd, 1H), 7.75 (d, 1H).
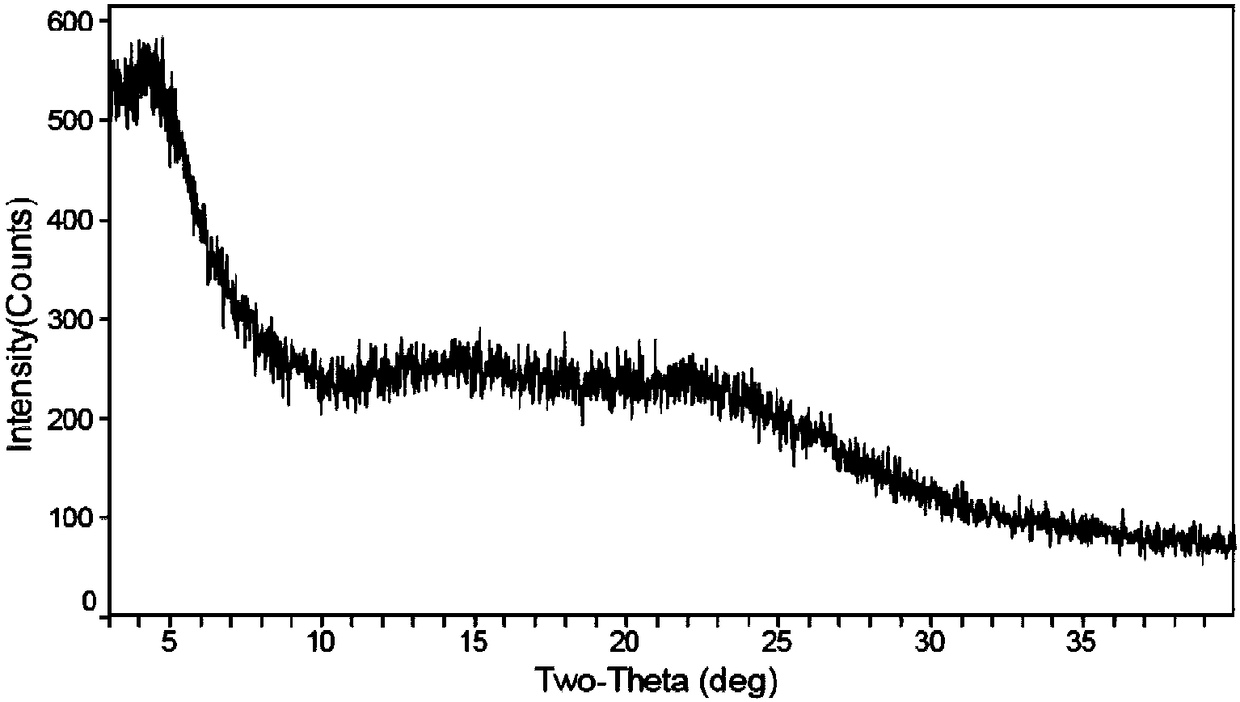
[0088] H-NMR spectrum and X-ray powder diffraction spectrum are shown in figure 1 with figure 2 .
Embodiment 2
[0089] Example 2: Antihypertensive efficacy test of QR01019K on spontaneously hypertensive rats
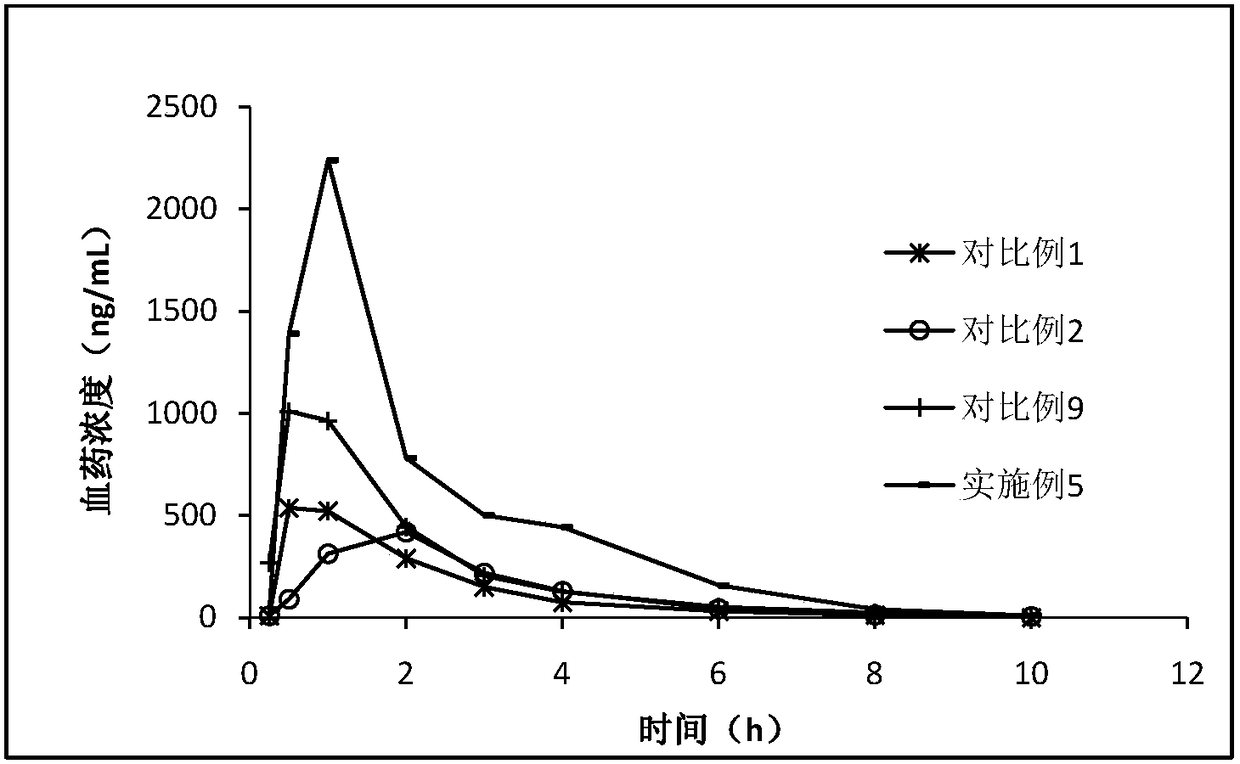
[0090] Spontaneously hypertensive rats aged 12 weeks (hereinafter referred to as SHR, purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.) were anesthetized by intraperitoneal injection of 2.5% sodium pentobarbital. The blood pressure sensing catheter was inserted into the abdominal aorta, the implant was fixed on the abdominal wall, and the postoperative daily care was performed after suturing. Animals whose systolic blood pressure exceeded 160mm Hg were selected into groups, with 8 animals in each group, 3 groups in total. The control group was given 0.5% sodium carboxymethylcellulose (hereinafter referred to as CMC-Na); the OR01019 group and the QR01019K group were dissolved in 0.5% CMC-Na, and the dosage was based on the effective dose of 1 mg / kg azilsartan. The drug volume is 4mL / kg, all administered by intragastric administration, with the systolic...
Embodiment 3
[0102] Embodiment 3: Preparation of oral solid preparation
[0103] Prepare the tablet of Preparation Example 1-8 according to the following preparation process, and the prescription is shown in Table 3 below.
[0104] Preparation:
[0105] (1) Preparation of adhesive: Dissolve the adhesive with pure water to obtain the corresponding aqueous solution for later use; take the pH regulator, add purified water above 80°C to dissolve completely, and cool to room temperature to obtain monosodium fumarate aqueous solution ; Mix the binder aqueous solution with the monosodium fumarate solution, and stir evenly to obtain the binder mixed solution.
[0106] (2) Granulation: take the active ingredient and excipients and mix them through a 40-mesh sieve, then pour them into a fluidized bed granulator, spray the prepared binder mixed solution, granulate the mixture, and Dry in a bed granulator, and pass the obtained granules through a 30-mesh sieve for granulation.
[0107] (3) Tablet c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Gross weight | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Sheet weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com