Non-irritating clarithromycin freeze-dried powder and preparation method thereof
A technology of clarithromycin and clarithromycin lipid, applied in the field of macrolide antibiotic preparation, can solve the problems affecting the clinical application and promotion of intravenous injections, high incidence of phlebitis, shrinking dosage form, etc. Insoluble, reduced dosing frequency, stable effect at room temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
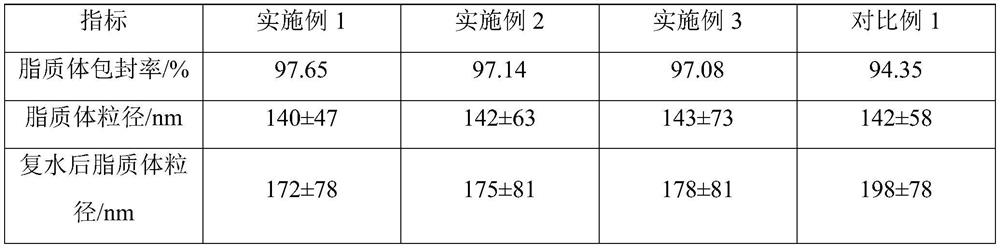
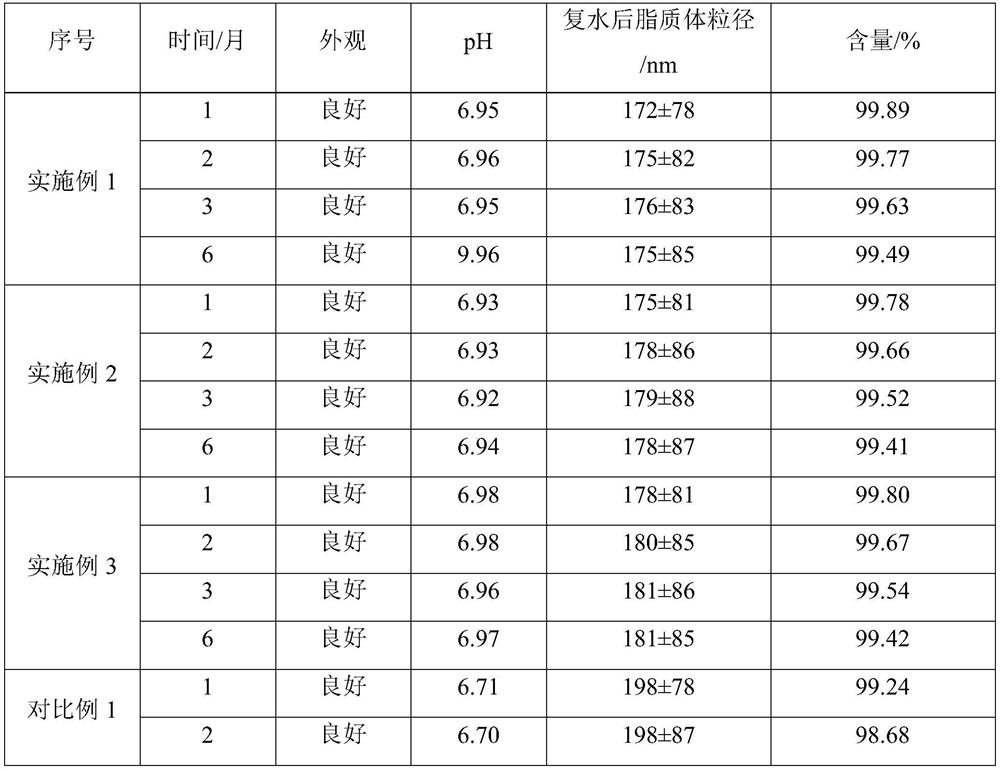
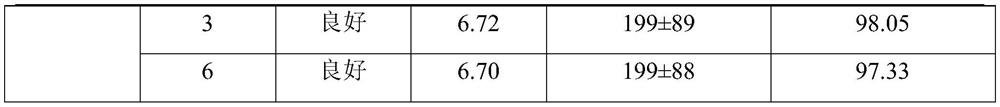
[0027] The non-irritating clarithromycin freeze-dried powder described in this example 1 is composed of clarithromycin liposome and a freeze-drying protective agent, and 25 g of the freeze-dried protective agent is added to each 100 mL of liposome solution; The plastid solution is prepared from the following raw materials: 2.0 g of clarithromycin, 2.0 g of sodium cholesteryl sulfate, 6.0 g of lecithin, 0.6 g of citric acid, 15 g of sucrose, 10 g of glucose, and the balance is phosphate buffer solution.
[0028] The preparation method of the non-irritating clarithromycin freeze-dried powder described in the present embodiment 1, consists of the following steps:
[0029] (1) Dissolve clarithromycin, sodium cholesteryl sulfate, lecithin and citric acid in absolute ethanol to obtain an oil phase, and keep the temperature at 70°C for 30min;
[0030] (2) the dehydrated alcohol in the oil phase is removed by rotary evaporation, and then blown dry with nitrogen to obtain a film;
[0...
Embodiment 2
[0038] The non-irritating clarithromycin freeze-dried powder described in this example 2 is composed of clarithromycin liposome and a freeze-drying protective agent, and 18 g of the freeze-dried protective agent is added to each 100 mL of liposome solution; The plastid solution is prepared from the following raw materials: 2.0 g of clarithromycin, 2.2 g of sodium cholesteryl sulfate, 6.2 g of lecithin, 0.5 g of citric acid, 10 g of sucrose, 8 g of glucose, and the balance is phosphate buffer solution.
[0039] The preparation method of the non-irritating clarithromycin freeze-dried powder described in the present embodiment 2 consists of the following steps:
[0040] (1) Dissolve clarithromycin, sodium cholesteryl sulfate, lecithin and citric acid in absolute ethanol to obtain an oil phase, and keep the temperature at 68°C for 35min;
[0041] (2) the dehydrated alcohol in the oil phase is removed by rotary evaporation, and then blown dry with nitrogen to obtain a film;
[004...
Embodiment 3
[0049]The non-irritating clarithromycin freeze-dried powder described in Example 3 is composed of clarithromycin liposome and a freeze-drying protective agent, and 30 g of a freeze-dried protective agent is added to each 100 mL of liposome solution; The plastid solution is prepared from the following raw materials: 2.0 g of clarithromycin, 2.2 g of sodium cholesteryl sulfate, 6.4 g of lecithin, 0.6 g of citric acid, 20 g of sucrose, 10 g of glucose, and the balance is phosphate buffer solution.
[0050] The preparation method of the non-irritating clarithromycin freeze-dried powder described in the present embodiment 3 consists of the following steps:
[0051] (1) Dissolve clarithromycin, sodium cholesterol sulfate, lecithin and citric acid in absolute ethanol to obtain an oil phase, and keep the temperature at 72°C for 33min;
[0052] (2) the dehydrated alcohol in the oil phase is removed by rotary evaporation, and then blown dry with nitrogen to obtain a film;
[0053] (3) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com