Non-irritating clarithromycin freeze-dried powder and preparation method thereof
A technology of clarithromycin and clarithromycin lipid, which is applied in the field of macrolide antibiotic preparation, can solve the problems affecting the clinical application and promotion of drug intravenous injections, high incidence of phlebitis, and poor tolerance of patients, and achieve the goal of overcoming Effects of almost insolubility, reduced number of administrations, and good appearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
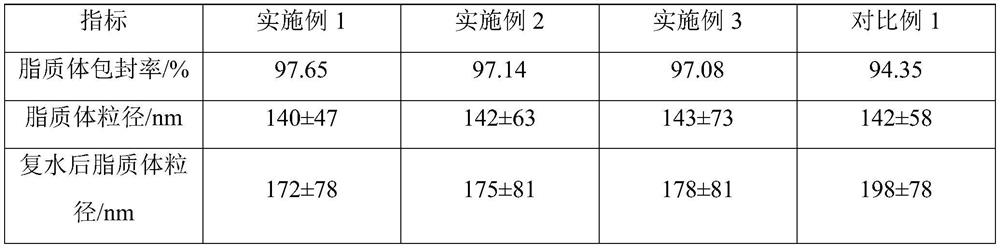
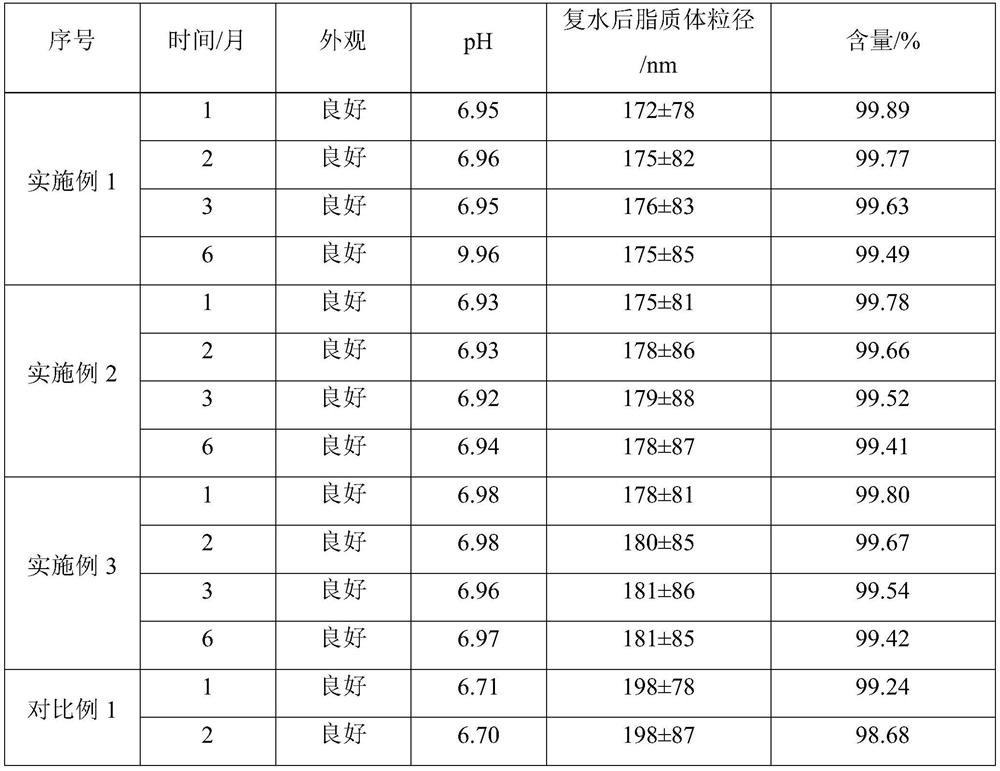
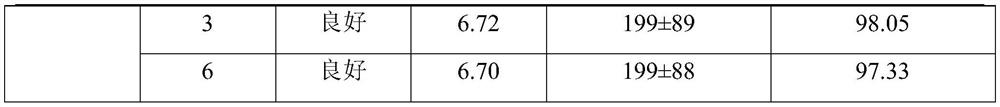
[0027] The non-irritating clarithromycin lyophilized powder described in Example 1 is composed of clarithromycin liposomes and a lyoprotectant, and 25g of the lyoprotectant is added to every 100mL liposome solution; the 100mL liposome The plastid solution is prepared from the following raw materials: clarithromycin 2.0g, sodium cholesteryl sulfate 2.0g, lecithin 6.0g, citric acid 0.6g, sucrose 15g, glucose 10g, and the balance is phosphate buffer solution.
[0028] The preparation method of the non-irritating clarithromycin lyophilized powder described in the present embodiment 1 consists of the following steps:
[0029] (1) Dissolve clarithromycin, sodium cholesteryl sulfate, lecithin and citric acid in absolute ethanol to obtain an oil phase, and keep the temperature at 70°C for 30 minutes;
[0030] (2) Rotary evaporation removes absolute ethanol in the oil phase, then blows dry with nitrogen to obtain a thin film;
[0031] (3) Keep the phosphate buffer solution with a pH v...
Embodiment 2
[0038] The non-irritating clarithromycin lyophilized powder described in Example 2 is composed of clarithromycin liposomes and a lyoprotectant, and 18g of the lyoprotectant is added to every 100mL liposome solution; the 100mL liposome The plastid solution is prepared from the following raw materials: clarithromycin 2.0g, sodium cholesteryl sulfate 2.2g, lecithin 6.2g, citric acid 0.5g, sucrose 10g, glucose 8g, and the balance is phosphate buffer solution.
[0039] The preparation method of the non-irritating clarithromycin lyophilized powder described in the present embodiment 2 consists of the following steps:
[0040] (1) Dissolve clarithromycin, sodium cholesteryl sulfate, lecithin and citric acid in absolute ethanol to obtain an oil phase, and keep the temperature at 68°C for 35 minutes;
[0041] (2) Rotary evaporation removes absolute ethanol in the oil phase, then blows dry with nitrogen to obtain a thin film;
[0042] (3) Keep the phosphate buffer solution with a pH va...
Embodiment 3
[0049]The non-irritating clarithromycin lyophilized powder described in Example 3 is composed of clarithromycin liposomes and a lyoprotectant, and 30g of the lyoprotectant is added to every 100mL liposome solution; the 100mL lipid The plastid solution is prepared from the following raw materials: clarithromycin 2.0g, sodium cholesteryl sulfate 2.2g, lecithin 6.4g, citric acid 0.6g, sucrose 20g, glucose 10g, and the balance is phosphate buffer solution.
[0050] The preparation method of the non-irritating clarithromycin freeze-dried powder described in the present embodiment 3 is made up of the following steps:
[0051] (1) Dissolve clarithromycin, sodium cholesteryl sulfate, lecithin and citric acid in absolute ethanol to obtain an oil phase, and keep the temperature at 72°C for 33 minutes;
[0052] (2) Rotary evaporation removes absolute ethanol in the oil phase, then blows dry with nitrogen to obtain a thin film;
[0053] (3) keep a phosphate buffer solution with a pH valu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com