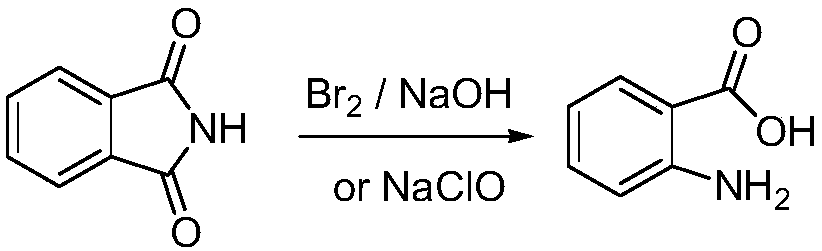Preparation method of 3,5-dibromo-2-aminobenzoic acid
An aminobenzoic acid and bromate technology, which is applied in the preparation of organic compounds, chemical instruments and methods, cyanide reaction preparation, etc., can solve the problems of inconvenient procurement and management, high production cost, high risk and the like of using enterprises, To achieve the effect of simple operation, stable purity and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Reaction Ⅰ: 6g (0.041mol) phthalimide, 3.67g (0.036mol) NaBr, 2.69g (0.018mol) NaBrO 3 Add it into 100g of water, stir to dissolve, add dropwise 6.64g (0.026mol) of 40% sulfuric acid, stir at room temperature for 12h, filter, and wash the solid with 2×20mL water to obtain a white solid of N-bromophthalimide.
[0040] Reaction II: Add N-bromophthalimide obtained in Reaction I to aqueous sodium hydroxide solution (15.6g, 0.39mol NaOH dissolved in 100g water) in batches at -5°C to 0°C, After the solid was completely dissolved, it was raised to room temperature and kept for 0.5 h.
[0041]Reaction Ⅲ: Add 50mL (0.36mol) of 50% sulfuric acid and 4.96g (0.048mol) of NaBr to the solution obtained in Reaction Ⅱ, cool down to -5°C to 0°C, and add dropwise 19.6g (0.098mol) of 17.1% peroxide Hydrogen aqueous solution, raised to room temperature to continue reaction for 12h after dripping, filtered, washed the solid with 2×20mL water, and dried to obtain 10.90g white solid of 3,5-d...
Embodiment 2
[0043] Reaction Ⅰ: 6g (0.041mol) phthalimide, 3.67g (0.036mol) NaBr, 2.69g (0.018mol) NaBrO 3 Add it into 100g of water, stir to dissolve, add dropwise 6.64g (0.026mol) of 40% sulfuric acid, stir at room temperature for 12h, filter, and wash the solid with 2×20mL water to obtain a white solid of N-bromophthalimide.
[0044] Reaction II: Add the N-bromophthalimide obtained in Reaction I to aqueous sodium hydroxide solution (15.6g, 0.39mol NaOH dissolved in 100g water) in batches at -5°C to 0°C. Solid After complete dissolution, it was raised to room temperature and incubated for 1 h.
[0045] Reaction Ⅲ: Add 61mL (0.73mol) of 36% hydrochloric acid and 4.96g (0.048mol) of NaBr to the solution obtained in reaction Ⅱ respectively, cool down to -5°C to 0°C, and add dropwise 19.6g (0.098mol) of 17.1% peroxide Hydrogen aqueous solution, raised to room temperature to react for 10 h after dripping, filtered, washed the solid with 2×20 mL of water, and dried to obtain 10.78 g of white ...
Embodiment 3
[0047] Reaction Ⅰ: 6g (0.041mol) phthalimide, 4.28g (0.036mol) KBr, 2.99g (0.018mol) KBrO 3 Add it into 100g of water, stir to dissolve, add dropwise 6.64g (0.026mol) of 40% sulfuric acid, stir at room temperature for 12h, filter, and wash the solid with 2×20mL water to obtain a white solid of N-bromophthalimide.
[0048] Reaction II: Add N-bromophthalimide obtained in Reaction I to aqueous sodium hydroxide solution (15.6g, 0.39mol NaOH dissolved in 100g water) in batches at -5°C to 0°C, After the solid was completely dissolved, it was raised to room temperature for 2 h.
[0049] Reaction Ⅲ: Add 50mL (0.36mol) of 50% sulfuric acid and 4.96g (0.048mol) of NaBr to the solution obtained in Reaction Ⅱ, cool down to -5°C to 0°C, and add dropwise 19.6g (0.098mol) of 17.1% peroxide Hydrogen aqueous solution, raised to room temperature to react for 12h after dropping, filtered, washed the solid with 2×25mL water, and dried to obtain 10.65g of white solid of 3,5-dibromo-2-aminobenzoic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com