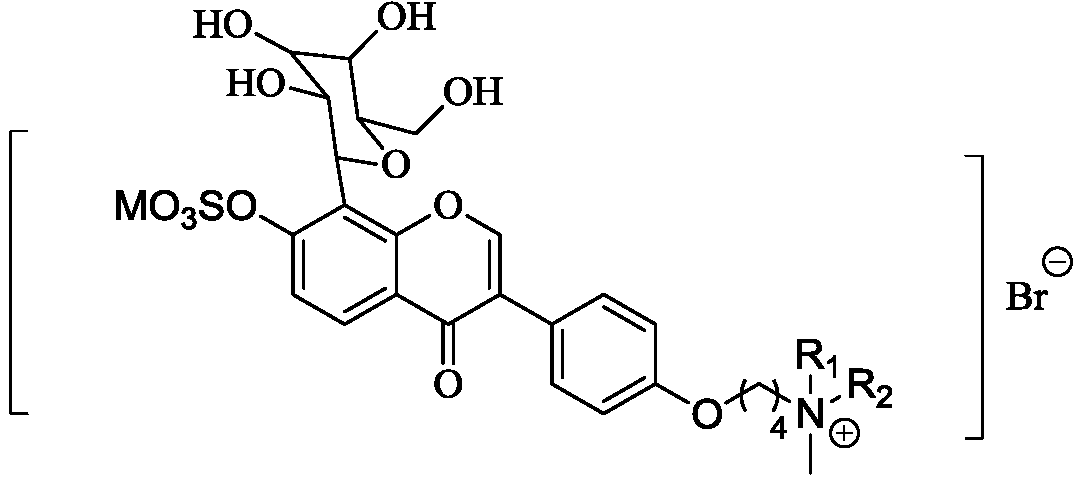
Puerarin derivative x with anti-platelet aggregation function and its preparation method and application
A technology of puerarin derivatives and operation methods, applied in the fields of organic chemistry, drug combination, blood diseases, etc., can solve the problems of low solubility of puerarin, affecting the pharmacology and efficacy of puerarin, and achieve good anti-platelet aggregation effect, Good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The preparation method of described puerarin derivative X, it comprises the following steps:
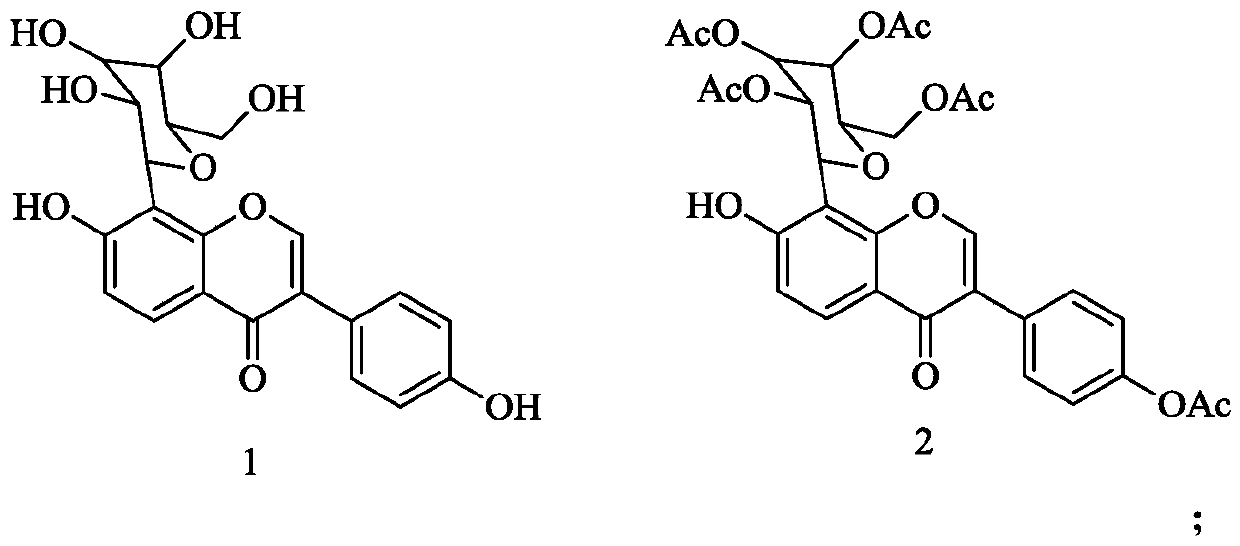
[0026] (1) Synthesis of Compound 2: Dissolving Compound 1 in pyridine, and reacting with acetic anhydride to obtain Compound 2; wherein the structural formulas of Compound 1 and Compound 2 are respectively:
[0027]
[0028] (2) Synthesis of compound 3: compound 2 obtained in step (1) is reacted with sulfur trioxide pyridinium salt and triethylamine to obtain compound 3; wherein, the structural formula of compound 3 is:
[0029]
[0030] (3) Synthesis of compound 4: react compound 3 obtained in step (2) with ammonia water to obtain compound 4; wherein the structural formula of compound 4 is:
[0031]
[0032] (4) Synthesis of compound 5: react compound 4 obtained in step (3) with 1,4-dibromobutane and MOH to obtain compound 5; wherein MOH is one of NaOH or KOH, the structural formula of compound 5 for:
[0033]
[0034] (5) Synthesis of puerarin derivative X: rea...
Embodiment 1
[0043] Embodiment one: the synthesis of compound 5:
[0044] (1) Preparation of compound 2:
[0045] Add 300 mL of dried pyridine to 50 g of compound 1 to completely dissolve compound 1, then add 100 g of acetic anhydride, stir at room temperature for 30 min, and place it at room temperature for 24 h to obtain mixture A. Slowly pour mixture A into ice water and stir thoroughly , a large amount of solids were precipitated, and solid A was obtained by suction filtration. Dissolve solid A with dichloromethane, add 5% aqueous sodium bicarbonate solution, stir for 60 min at room temperature, separate liquids with a separatory funnel and collect the organic layer, and recover the organic layer under reduced pressure The solvent was dried to obtain solid B, and solid B was subjected to silica gel column chromatography, and a gradient was carried out with petroleum ether-ethyl acetate as the eluent. According to the volume ratio of petroleum ether and ethyl acetate, it was 45:1, 35:1,...
Embodiment 2
[0054] Embodiment 2: Puerarin derivative X 1 Preparation of:
[0055] Put 0.25mmol of compound 5 obtained in Example 1 into a reaction flask, add 500mL of absolute ethanol, reflux and stir at 85°C for 45min, then add 0.3mmol of N-methyldi-n-octylamine, and continue the reflux reaction for 24h. After cooling, ethanol was recovered under reduced pressure to dryness to obtain a light yellow solid powder. The light yellow solid powder was separated and purified by silica gel column chromatography, using chloroform-methanol as the eluent, according to the volume ratio of chloroform and methanol: 50:1, 45:1, 40:1, 35:1, 25:1, 15:1, 10:1, 5:1 elution sequence for gradient elution, the volume ratio of collected chloroform to methanol is 5:1 When the eluted part, the collected eluted part was removed under reduced pressure to obtain a light yellow solid powder, which is the puerarin derivative X 1 . At room temperature, puerarin derivative X 1 The solubility in water is about 362mg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com