A kind of puerarin derivative b and its preparation method and application
A technology of puerarin derivatives and puerarin, applied in organic chemistry methods, drug combinations, organic chemistry, etc., can solve the problems of puerarin's poor water solubility and fat solubility, low bioavailability, and allergic reactions, etc., and achieve good results Anti-platelet aggregation effect, simple synthesis method, good water solubility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
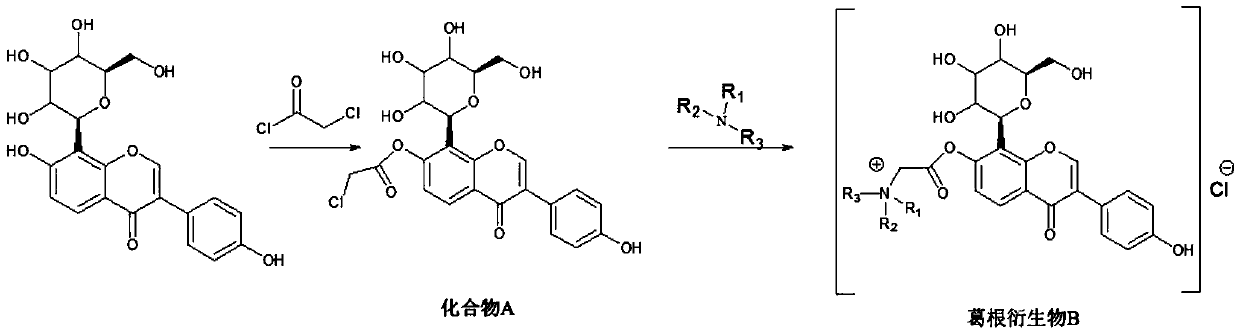
[0020] The preparation method of described puerarin derivative B, it comprises the following steps:
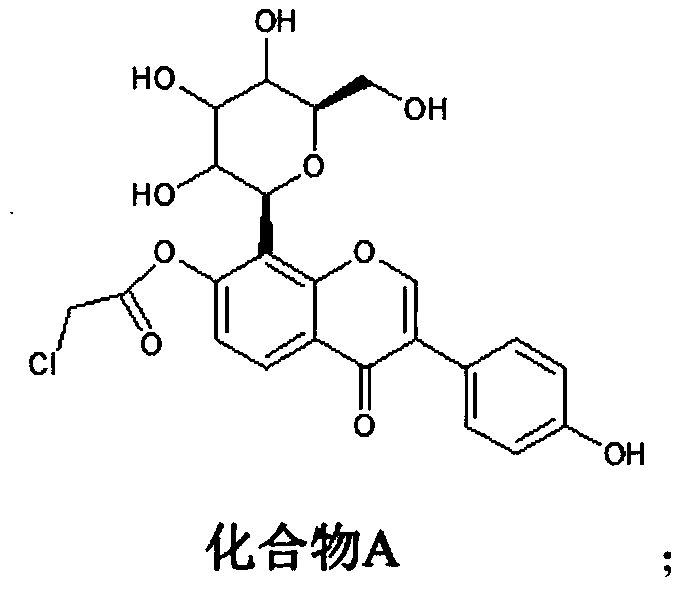
[0021] (1) Synthesis of compound A: react puerarin with chloroacetyl chloride to obtain compound A, wherein the structural formula of compound A is:
[0022]
[0023] (2) Synthesis of the target compound puerarin derivative B: react the compound A obtained in step (1) with a tertiary amine to obtain the described puerarin derivative B; wherein, the tertiary amine is triethylamine, N- One of methyl dioctylamine and N-methyl didecylamine.
[0024] Wherein, the specific operation method of the step (1) is: dissolve puerarin in the organic solvent A, add an organic base, control the temperature of the system at 0-5 ° C, slowly add the organic solvent A of chloroacetyl chloride dropwise, drop In the process of adding chloroacetyl chloride, the temperature of the control system is no more than 5°C; after the addition of chloroacetyl chloride is completed, the temperature of the ...
Embodiment 1
[0033] Synthesis of Compound A: Dissolve 4.16g (0.01mol) of puerarin in chloroform, add 1.19g of pyridine (0.015mol), control the temperature of the system at 0°C, and slowly add dropwise a chloroform solution of 1.36g of chloroacetyl chloride (0.012mol) , during the process of dropping chloroacetyl chloride, control the temperature of the system not to exceed 5°C. After the dropwise addition of chloroacetyl chloride is completed, slowly raise the temperature of the system to 28°C, and react at 28°C for 22 hours. During the reaction, use TLC followed the reaction process, and the raw material point basically disappeared after 22 hours of reaction. Afterwards, the reaction solution was concentrated to obtain a concentrate, which was purified by silica gel column chromatography (eluent: petroleum ether / ethyl acetate=10:1) to obtain 3.57 g of compound A with a yield of 72.4%.
[0034] NMR analysis: 1 H-NMR(400MHz,DMSO-d6):8.42(1H,s),7.99(1H,d),7.43(1H,d),7.35(2H,m),6.99(2H,m),5....
Embodiment 2
[0036] Synthesis of Compound A: Dissolve 4.16g (0.01mol) of puerarin in chloroform, add 0.791g of pyridine (0.01mol), control the temperature of the system at 2°C, and slowly add dropwise a chloroform solution of 1.13g of chloroacetyl chloride (0.01mol) , during the process of dropping chloroacetyl chloride, the temperature of the system is controlled not to exceed 5°C. After the dropwise addition of chloroacetyl chloride is completed, the temperature of the system is slowly raised to 25°C, and the reaction is carried out at 25°C for 24 hours. During the reaction, use TLC followed the reaction process, and the raw material point basically disappeared after 24 hours of reaction. Afterwards, the reaction solution was concentrated to obtain a concentrate, which was purified by silica gel column chromatography (eluent: petroleum ether / ethyl acetate=10:1) to obtain 3.32 g of compound A with a yield of 67.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com