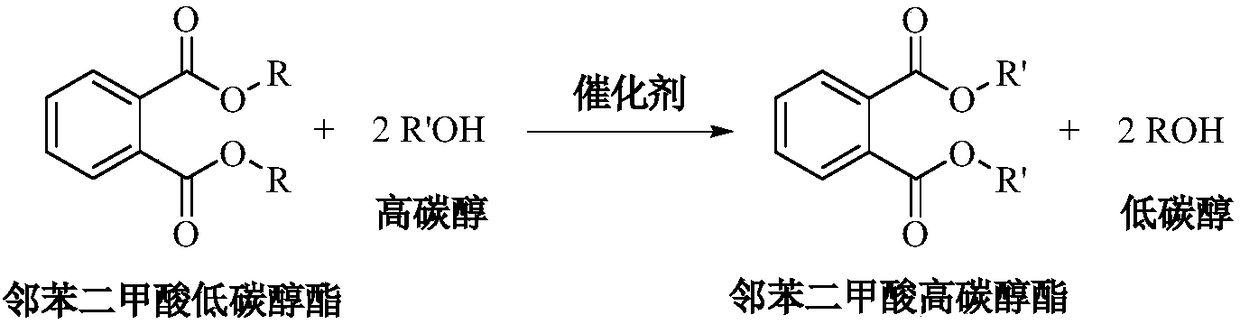
Method for preparing high-carbon alcohol phthalate ester through ester exchange continuous reaction
A technology of phthalic acid and high-carbon alcohol ester, which is applied in the preparation of ester group and hydroxyl reaction, chemical instruments and methods, metal/metal oxide/metal hydroxide catalyst, etc., can solve the problem of high raw material consumption and raw material consumption. It can achieve the effects of good storage and transportation stability, clean process and energy saving, and low consumption of raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
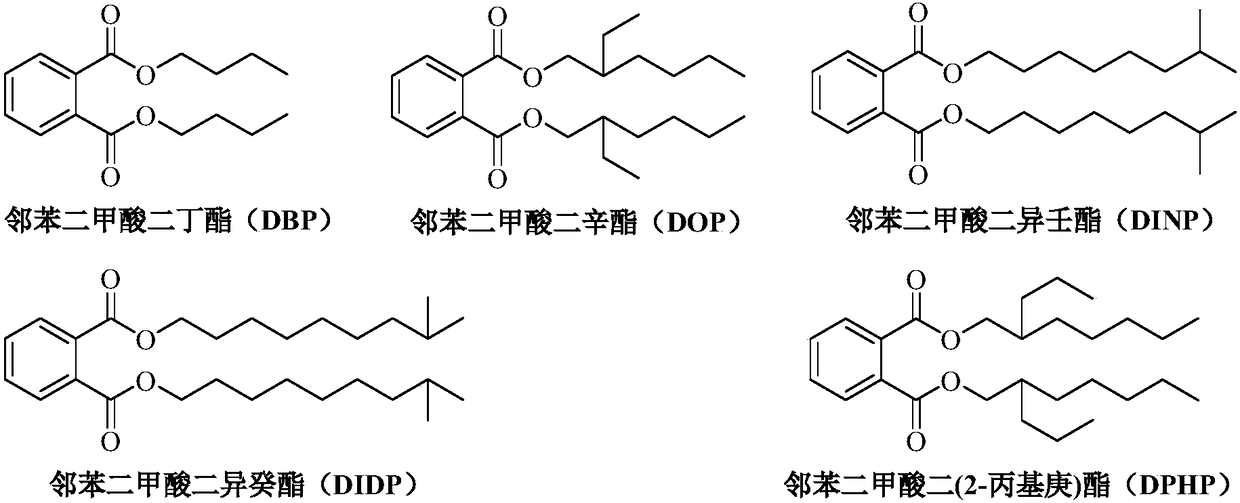
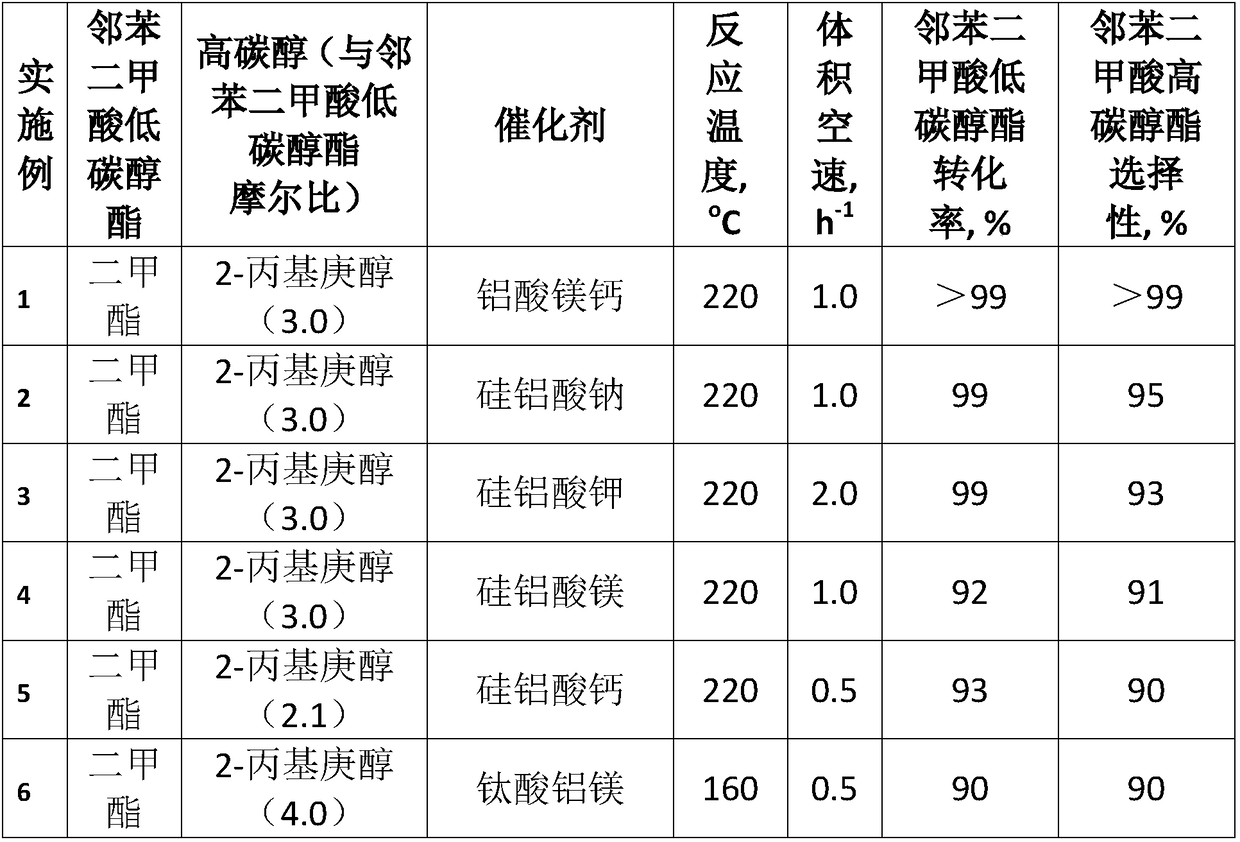
[0021] Load 100ml calcium magnesium aluminate catalyst into the fixed bed reactor, after the temperature of the reaction tube rises to 220°C, pump in the 2-propylheptanol / dimethyl phthalate (DMP) mixture with a molar ratio of 3 , flow rate setting 100ml / h (volume space velocity 1h -1 ), after the reaction runs continuously for 4h, sampling is carried out by gas chromatography-mass spectrometry for qualitative and quantitative analysis, the conversion rate of raw material DMP and the selectivity of product DPHP all reach more than 99%. After the reaction was continuously run for 24 hours, another sample was taken for analysis, and the conversion rate of DMP and the selectivity of DPHP were both above 99%.
Embodiment 2~10
[0023] The specific method of Examples 2-10 is similar to that of Example 1, and the specific reaction conditions and reaction results after continuous operation for 24 hours are shown in Table 1. Sodium aluminosilicate, potassium aluminosilicate, magnesium aluminosilicate, calcium aluminosilicate, aluminum magnesium titanate, magnesium calcium titanate, aluminum magnesium zirconate, magnesium calcium aluminate as catalyst, dimethyl phthalate , diethyl phthalate, dibutyl phthalate and other low-carbon alcohol esters of phthalates undergo transesterification reactions with high-carbon alcohols such as 2-propylheptanol, isononanol, and isodecyl alcohol. The molar ratio of carbon alcohol to low-carbon alcohol phthalate is 2.1-4.0, the reaction temperature is 160-220°C, and the volume space velocity is 0.5-2h -1 , the conversion rate of low-carbon alcohol phthalate and the selectivity of high-carbon alcohol phthalate can reach more than 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com