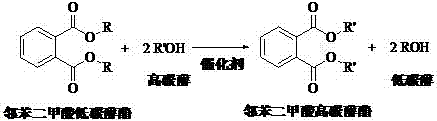
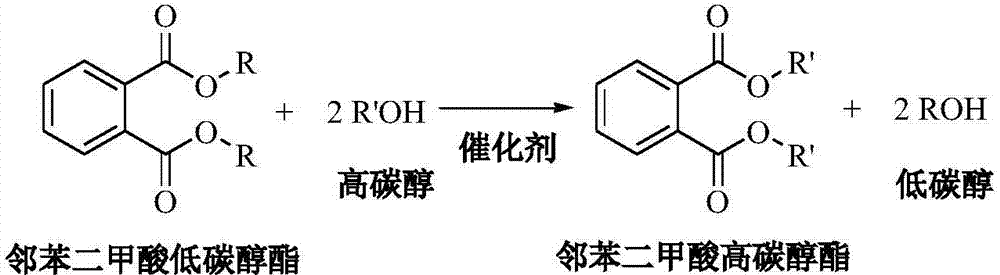
A kind of method that transesterification prepares phthalic acid higher carbon alcohol ester
A technology of dimethyl phthalate and phthalic acid, which is applied in the field of transesterification to prepare higher alcohol phthalate, can solve the problems of many side reactions, less side reactions, low consumption of raw materials, etc. Low corrosiveness, mild reaction conditions, clean process and energy saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 19.4 grams of dimethyl phthalate (DMP for short, 0.1 mol), 47.5 grams of 2-propylheptanol (0.3 mol), and 0.2 grams of potassium titanium oxalate (1.0% of the mass of DMP) into 100 ml of In the two-necked round-bottomed flask of the water dispenser, connect the condenser to the water separator, stir evenly, and heat up to 200 ° C for 5 hours. After the reaction, the mixture is qualitatively analyzed by gas chromatography-mass spectrometry, and quantitatively analyzed by gas chromatography. Both the conversion rate of DMP and the selectivity of DPHP are above 99%.
Embodiment 2
[0024] Catalyst blank experiment: no catalyst was added, other conditions were the same as in Example 1, the conversion rate of DMP only reached 50%, and the selectivity of DPHP was only 5%.
Embodiment 3~21
[0026] The specific method of Examples 3-21 is similar to that of Example 1, and the specific reaction conditions and results are shown in Table 1.
[0027] It can be seen from the catalyst blank experiment that DMP and 2-propylheptanol were heated and reacted at 200° C. for 5 hours, and the conversion rate of DMP and the selectivity of DPHP were all very low (embodiment 2). Concentrated sulfuric acid has a good catalytic effect on the preparation of DPHP by the esterification reaction of 2-propylheptanol and phthalic anhydride, and the conversion rate of phthalic anhydride and the selectivity of DPHP can reach more than 99%. Yet, the vitriol oil is used to catalyze the transesterification reaction of DMP and 2-propylheptanol, although the DMP conversion rate can reach more than 99%, but the DPHP selectivity only has 80% (embodiment 3). Trying to use tin titanate as a catalyst, the reaction result under the same conditions is the same as that of the catalyst blank experiment (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com