Amlodipine besylate tablet and preparation method thereof
A technology of amlodipine besylate tablets and amlodipine besylate, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve the problem of unstable dissolution and taking by patients. Side effects, instability and other problems, to achieve excellent stability, good compressibility and fluidity, and improve the effect of dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
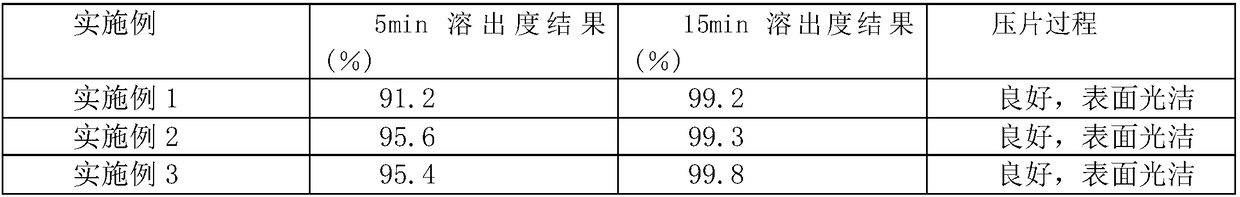
Embodiment 1
[0023] A kind of amlodipine besylate tablet, by tablet gross weight percentage, is made up of following composition: 15 parts of amlodipine besylate, 40 parts of microcrystalline cellulose, 13 parts of L-tyrosine, 25 parts of solubilizing agent 15 parts of disintegrant, 8 parts of crospovidone, 3 parts of lubricant, 10 parts of excipient, 30 parts of ethanol.
[0024] Wherein, the disintegrant is carboxymethyl starch sodium; the excipient is a mixture of carmellose sodium and polyethylene glycol 400 in a mass ratio of 2:5; the solubilizer is anhydrous calcium hydrogen phosphate, solubilizing Tween -80. A mixture of sodium lauryl sulfate in a mass ratio of 1:3:5; the lubricant is magnesium stearate; the pH value of microcrystalline cellulose is 6.5.
[0025] A kind of preparation method of amlodipine besylate sheet, described method comprises the steps:
[0026] (1) After weighing 15 parts of amlodipine besylate, 40 parts of microcrystalline cellulose, 13 parts of L-tyrosine, ...
Embodiment 2
[0030] A kind of amlodipine besylate tablet, by tablet gross weight percentage, is made up of following composition: 20 parts of amlodipine besylate, 30 parts of microcrystalline cellulose, 10 parts of L-tyrosine, 15 parts of solubilizing agent 20 parts of disintegrant, 10 parts of crospovidone, 2 parts of lubricant, 8 parts of excipient, 40 parts of ethanol.
[0031] Among them, the disintegrant is carmellose sodium; the excipient is a mixture of carmellose sodium and polyethylene glycol 400 in a mass ratio of 2:5; A mixture of Wen-80 and sodium lauryl sulfate in a mass ratio of 1:3:5; the lubricant is talc; the pH value of microcrystalline cellulose is 7.
[0032] The present invention also provides a kind of preparation method of amlodipine besylate tablet, and method comprises the steps:
[0033] (1) After weighing 20 parts of amlodipine besylate, 30 parts of microcrystalline cellulose, 10 parts of L-tyrosine, 15 parts of solubilizer, and 10 parts of crospovidone by weigh...
Embodiment 3
[0037] A kind of amlodipine besylate tablet, by tablet gross weight percentage, is made up of following composition: 30 parts of amlodipine besylate, 60 parts of microcrystalline cellulose, 20 parts of L-tyrosine, 20 parts of solubilizing agent 5 parts, 30 parts of disintegrant, 5 parts of crospovidone, 5 parts of lubricant, 5 parts of excipient, 50 parts of ethanol.
[0038] Wherein, the disintegrant is hydroxypropyl cellulose; the excipient is a mixture of carmellose sodium and polyethylene glycol 400 in a mass ratio of 2:5; the solubilizer is anhydrous calcium hydrogen phosphate, solubilizing Tween -80. A mixture of sodium lauryl sulfate in a mass ratio of 1:3:5; the lubricant is silicon dioxide; the pH value of the microcrystalline cellulose is 6.8.
[0039] The present invention also provides a kind of preparation method of amlodipine besylate tablet, described method comprises the steps:
[0040] (1) After weighing 30 parts of amlodipine besylate, 60 parts of microcryst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com