BODIPY derivative BDO-N3 and synthetic method and usage thereof
A BDP-N3, fluoroboron dipyrrole technology is applied in the field of fluoroboron dipyrrole derivatives to achieve the effects of obvious detection signal, convenient operation and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
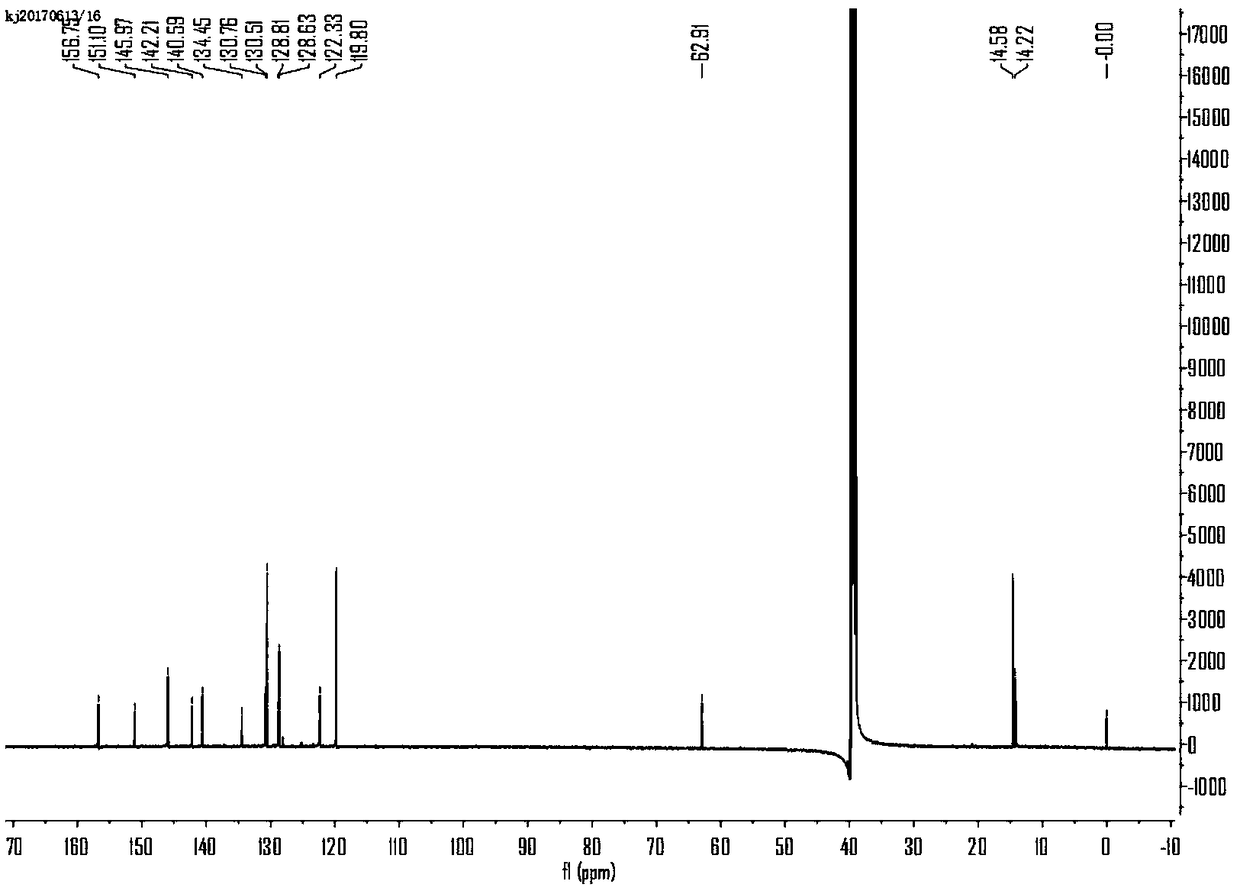
[0037] BDP-N 3 Preparation and Characterization of
[0038] 1) Stir 4-pyridinecarbaldehyde (9.0mmol, 0.96g) and 2,4-dimethylpyrrole (19.4mmol, 1.85g) in deoxymethylene chloride (150ml). A drop of trifluoroacetic acid was added and the mixture was stirred at room temperature under argon. The combined solution was treated with dichlorodicyanobenzoquinone (9.0 mmol, 2.04 g), stirring was continued for 4 hours, then triethylamine (15 mL) was added. Add BF after 15 minutes 3 ·Et 2O (15 mL), and the mixture was stirred at room temperature for 3 hours, and after washing with saturated sodium bicarbonate solution, the organic phase was separated, dried over anhydrous sodium sulfate, filtered and concentrated. The residue was subjected to silica gel column chromatography (CH 2 Cl 2 / petroleum ether, 1 / 1, V / V, eluting) purification, obtain desired compound 1 as red powder (0.35g, 12%);
[0039] 2) Compound 1 (0.06 mmol, 0.02 g) and 1-azido-4-(bromomethyl)benzene (0.6 mmol, 0.10 g...
Embodiment 2
[0042] In a cuvette, add 2 mL of DMSO, 0.5 μM BDP-N 3 DMSO solution, add hydrogen sulfide solution in volumes of 0, 4, 8, 12, 16, 20, 24, and 28 μL respectively, and measure the fluorescence intensity at 515 nm on a fluorescence spectrometer after 15 minutes as 125.8, 213.7, 342.1, 438.2, 579.4, 714.3, 837.6, 967.9, the fluorescence intensity gradually increased. Fluorescence emission diagram see Figure 4 .
Embodiment 3
[0044] Prepare a PBS buffer solution with a pH=7.4 and a concentration of 10mM, prepare 2mM BDP-N 3 DMSO solution, prepare 0.2M hydrogen sulfide aqueous solution; in the fluorescence cuvette, add 2mL of DMSO and 0.5μM BDP-N 3 DMSO solution, and then add 10 times the equivalent of other analytes and hydrogen sulfide: SO 3 2- ,S 2 o 3 2- ,SCN - ,PO 4 3- ,NO 3 - ,NO 2 - , HSO 3 - , HCO 3 - , AcIO - ,F - , Cl - ,Br - , I - ,,CO 3 2- ,SCN - , SO 4 2- ,ClO - , Cys, GSH, and the aqueous solution of Hcy are detected on a fluorescence spectrophotometer, and the fluorescence intensity histogram at 515nm corresponding to different analytes is drawn (see Figure 5 ). Hydrogen sulfide makes the fluorescence intensity of the detection system increase significantly at 515nm, and other analytes basically do not cause changes in the fluorescence intensity of the detection system.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com