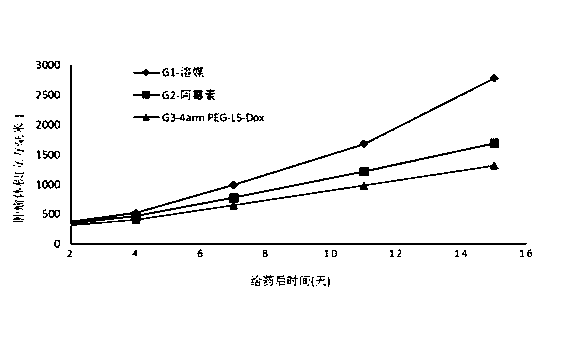
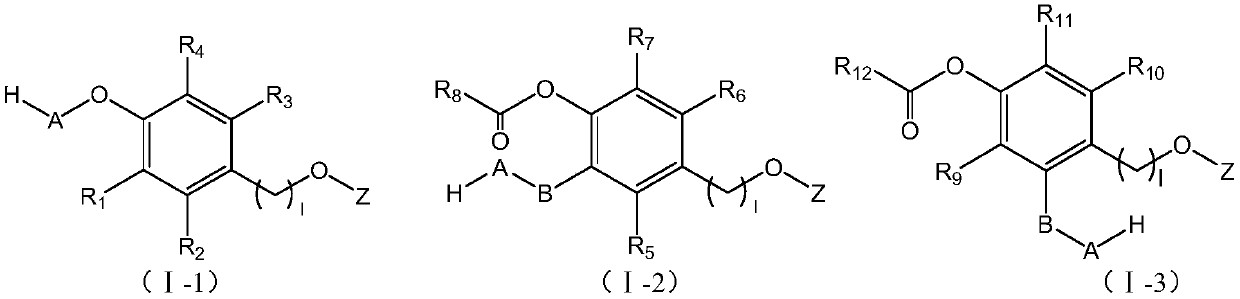
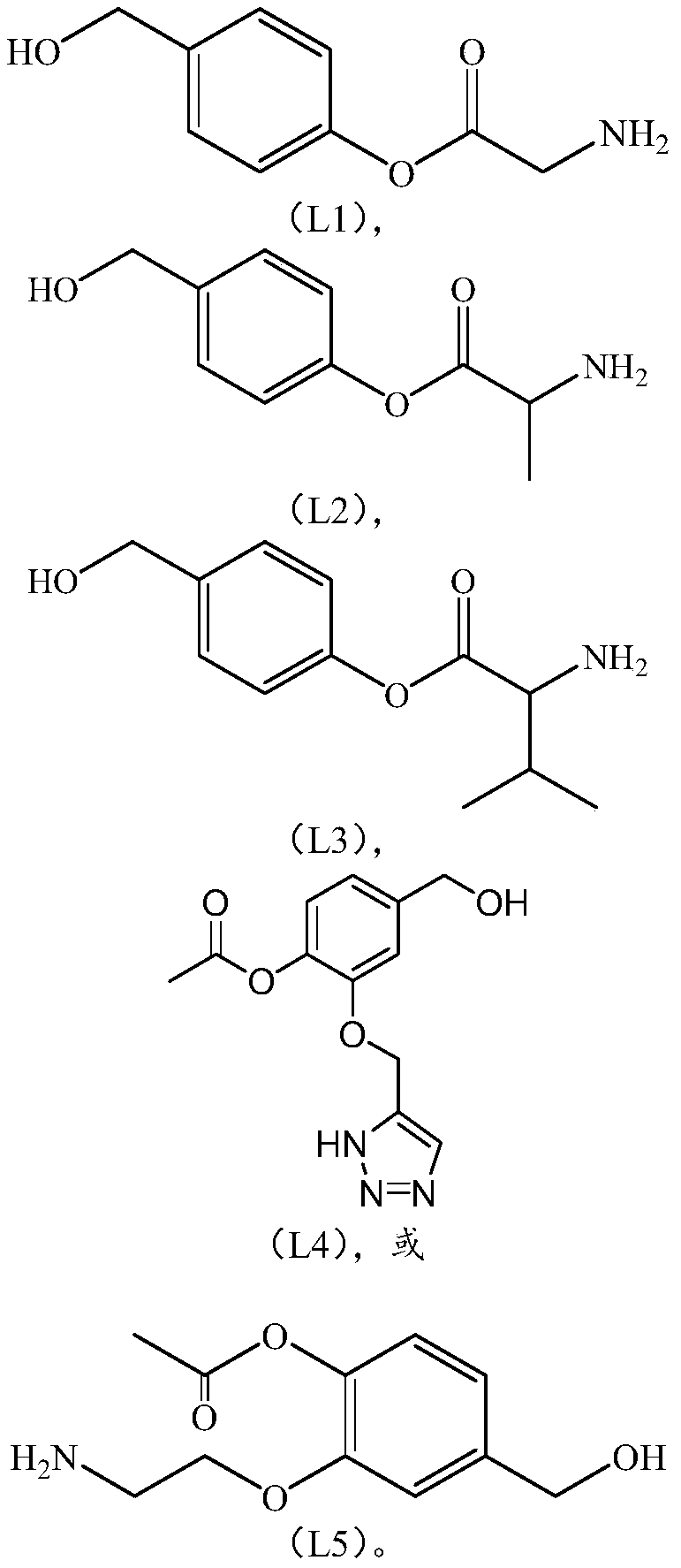
Linker compound, polyethylene glycol-linker conjugate and derivative thereof, and polyethylene glycol-linker-drug conjugate
A technology of linker conjugates and polyethylene glycol, applied in the field of medicine, can solve the problems of reduced drug efficacy, prolonged physiological half-life of drugs, and rapid occurrence of adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0181] The synthesis of embodiment 1 connecting chain (L)
[0182]
[0183] Add BOC-amino acid (92.2mmol) and N,N-dicyclohexylcarbodiimide (DCC, 23.8g, 115.3mmol) into dichloromethane (500mL), cool in an ice-water bath, then add p-hydroxybenzyl alcohol ( 11.4 g, 92.2 mmol), the ice bath was removed after the addition, and the reaction was carried out overnight at room temperature. After filtration, the filter cake was washed with ethyl acetate, and the filtrate was evaporated to dryness to obtain a crude product, which was purified by column chromatography to obtain product 1.
[0184] 1a: 19.7g, yield 76.0%. 1 H NMR: (CDCl 3 ): 8.75(s,1H), 7.22(d,2H), 7.05(d,2H), 4.87(s,2H), 3.74(s,2H), 1.52(s,9H).
[0185] 1b: 20.3g, yield 74.8%. 1 H NMR: (CDCl 3 ):8.74(s,1H),7.21(d,2H),7.05(d,2H),4.88(s,2H),3.77(m,1H),1.51(s,9H),1.27(d,3H) .
[0186] 1c: 21.6g, yield 72.5%. 1 H NMR: (CDCl 3 ):8.75(s,1H),7.22(d,2H),7.05(d,2H),4.87(s,2H),3.61(d,1H),2,82(m,1H),1.52(s, 9H), 1.06(d,...
Embodiment 2
[0191] Synthesis of the conjugate (mPEG-L (40K)) of embodiment 2 monomethoxypolyethylene glycol acetic acid and connecting chain
[0192]
[0193] Monomethoxypolyethylene glycol-acetic acid (mPEG-CM, 40K, 5g, 0.125mmol), compound L (0.25mmol, prepared in Example 1)
[0194] Add 1-hydroxybenzotriazole (HOBt, 16.9mg, 0.125mmol) into the reaction flask, dissolve with dichloromethane, then add diisopropylethylamine (45.2mg, 0.35mmol), stir well, After cooling in an ice bath, (EDCI, 47.9 mg, 0.25 mmol) was added in batches. After the addition, the system naturally rose to room temperature and reacted overnight. After concentrating the next day, the residue was crystallized with isopropanol, filtered with suction, and dried to obtain the product mPEG-L.
[0195] mPEG-L1 (40K): 4.6 g, yield 92.4%.
[0196] mPEG-L2 (40K): 4.5g, yield 90.8%.
[0197] mPEG-L3 (40K): 4.7g, yield 93.7%.
Embodiment 3
[0198] Embodiment 3 Preparation of monomethoxy polyethylene glycol-doxorubicin conjugate (mPEG-L-Dox (40K))
[0199]
[0200] Compound mPEG-L (0.075mmol, prepared in Example 2) was added to the reaction flask, dissolved with dichloromethane (30mL), N 2 Cool under protection, add succinimide carbonate (23.0 mg, 0.09 mmol), stir to dissolve, then add triethylamine (10.1 mg, 0.1 mmol), remove the cooling bath after the addition, and react overnight at room temperature. The reaction solution was concentrated, and the residue was crystallized with isopropanol to obtain the product mPEG-L-NHS.
[0201] mPEG-L1-NHS (40K): 2.6g, yield 88.5%.
[0202] mPEG-L2-NHS (40K): 2.7g, yield 89.2%.
[0203] mPEG-L3-NHS (40K): 2.6g, yield 87.9%.
[0204] The compound mPEG-L-NHS (0.06mmol, prepared in the above steps) was dissolved in dichloromethane (25mL), N 2 Cool under protection, add diisopropylethylamine (12.9 mg, 0.1 mmol), stir well, then add doxorubicin hydrochloride (52.2 mg, 0.09...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com