Medical degradable magnesium alloy suture and preparation method thereof
A magnesium alloy and suture technology, which is applied in the field of medical degradable magnesium alloy suture and its preparation, can solve the problems of unsatisfactory suture effect, easy breakage, suture failure, etc., to facilitate clinical suture operation, inhibit wound infection, The effect of improving usability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
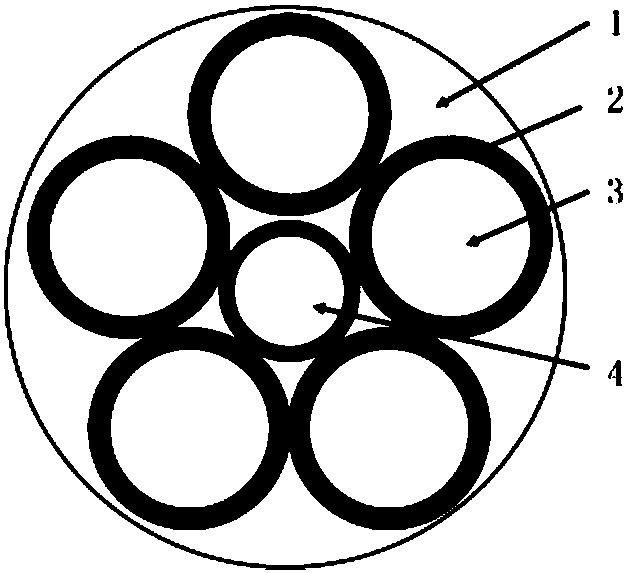
[0026] as attached figure 1 As shown, a medical degradable magnesium alloy suture is composed of five pure magnesium wires with a diameter of 0.52 mm and one pure magnesium wire with a diameter of 0.36 mm as the core, and degradable gelatin as the outer surface layer. , and its specific preparation process is as follows:
[0027] 1) Prepare pure magnesium into pure magnesium round wires with diameters of 0.52mm and 0.36mm;
[0028] 2) Perform fluoridation treatment on the pure magnesium wire obtained in step 1), select a solution whose main components are 2g / L sodium fluoride, 2g / L potassium fluoride and hydrofluoric acid, immerse the pure magnesium wire in the solution, soak For 12 hours, a dense porous ceramic layer rich in magnesium fluoride is formed on the surface in situ, with an average layer thickness of 5 μm;
[0029] 3) Take 5 pieces of 0.52mm diameter pure magnesium wire obtained in step 2) and 1 piece of 0.36mm diameter pure magnesium wire obtained in step 2), pl...
Embodiment 2
[0034] A medical degradable magnesium alloy suture, which is composed of four AZ61 magnesium alloy wires with a circumscribed circle diameter of 0.1 mm and a triangular cross-section wound as the core with a porous ceramic layer on the surface, and degradable polylactic acid as the outer surface layer. The specific preparation process as follows:
[0035] 1) Prepare the AZ61 magnesium alloy into a triangular wire with a circumcircle diameter of 0.1 mm;
[0036] 2) Perform micro-arc oxidation treatment on the AZ61 magnesium alloy wire obtained in step 1). The main components of the electrolyte are 13g / L sodium silicate, 5g / L sodium phosphate and 2.5g / L sodium hydroxide. AZ61 magnesium alloy wire is immersed in the electrolyte, and a pulse voltage of 380V is applied for 3 minutes to make the surface original. Form a dense porous ceramic layer rich in magnesium oxide, magnesium silicate, and magnesium phosphate, with an average layer thickness of 6 μm;
[0037] 3) Take 4 AZ61 m...
Embodiment 3
[0042] A medical degradable magnesium alloy suture, which is composed of seven Mg2Zn magnesium alloy wires with a diameter of 0.03 mm and a porous ceramic layer on the surface as the core, and degradable polyamide as the outer surface layer. The specific preparation process is as follows:
[0043] 1) Prepare the Mg2Zn magnesium alloy into a round wire with a diameter of 0.03mm;
[0044] 2) Perform micro-arc oxidation treatment on the Mg2Zn magnesium alloy wire obtained in step 1). The main components of the electrolyte are 10g / L sodium silicate, 3g / L sodium phosphate, 2g / L sodium hydroxide and 5g / L hydroxyapatite nanoparticles, and the Mg2Zn magnesium alloy wire is immersed in the electrolyte, and pulsed The voltage is 400V, and the time is 2 minutes, so that the dense porous ceramic layer rich in magnesium oxide, magnesium silicate, magnesium phosphate, and hydroxyapatite is formed on the surface in situ, with an average layer thickness of 4 μm;
[0045]3) Take 7 Mg2Zn magne...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com