A kind of regenerable catalyst for removing organic chlorine and preparation method thereof
A catalyst and organochlorine technology, applied in chemical instruments and methods, physical/chemical process catalysts, heterogeneous catalyst chemical elements, etc., can solve problems such as low dynamic adsorption rate, limited adsorption capacity, and short service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
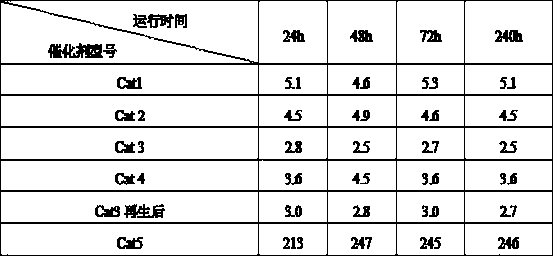
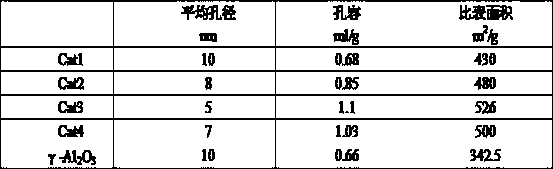
[0019] 197g aluminum sol (Al 2 o 3 The content of 22%), 33.3g silica sol (SiO 2 content of 30%), 38.1g of nickel nitrate, 2.5g of tetraethylammonium bromide and 300g of water, stirred for 6 hours, and mixed evenly to obtain the initial sol-gel mixture. Crystallize for 8 hours. The pH value of the crystal slurry was adjusted to neutral with 0.5mol / L urea, and 4.28g ammonium molybdate, 59.7g copper nitrate and 6.3g cerium nitrate were added, and the ion exchange reaction was carried out for 8h. After the reaction is completed, the above slurry is added to a flocculant for precipitation, filtered, washed, dried, extruded, and the filtered liquid is reused for the next reaction. After drying at 150°C for 2 hours and calcining at 500°C for 6 hours, a regenerable adsorption dechlorination catalyst Cat1 was obtained.
Embodiment 2
[0021] 179g of aluminum sol, 10g of silica sol, 44.6g of magnesium chloride, 15.1g of nickel nitrate, 2.5g of cetyltrimethylammonium bromide and 300g of water were stirred for 10 hours and mixed evenly to obtain the initial sol-gel mixture, and the initial sol-gel The glue material was moved into a synthesis kettle and sealed, and crystallized at 150 for 8 hours. The pH value of the crystal slurry was adjusted to neutral with 0.5mol / L urea, and 6.26g of ammonium molybdate, 59.7g of copper nitrate and 6.3g of cerium nitrate were added, and the ion exchange reaction was carried out for 28h. After the reaction is completed, the above slurry is added to a flocculant for precipitation, filtered, washed, dried, extruded, and the filtered liquid is reused for the next reaction. After drying at 120°C for 4 hours and calcining at 500°C for 6 hours, a regenerable adsorption dechlorination catalyst Cat2 was obtained.
Embodiment 3
[0023] 192.5g of aluminum sol, 23g of silica sol, 33g of magnesium chloride, 26.6g of nickel nitrate, 2.5g of tetraethylammonium bromide and 300g of water were stirred for 6 hours and mixed evenly to obtain the initial sol-gel mixture, and the initial sol-gel material was transferred to the synthetic The kettle was sealed and crystallized at 220°C for 20 hours. Use 0.5mol / L urea to adjust the pH value of the crystal slurry to neutral, add 4.28g ammonium molybdate, 48.7g copper nitrate and 5.1g cerium nitrate, and carry out ion exchange reaction for 20h. After the reaction is completed, the above slurry is added to a flocculant for precipitation, filtered, washed, dried, extruded, and the filtered liquid is reused for the next reaction. After drying at 120°C for 3 hours and calcining at 600°C for 5 hours, a regenerable adsorption dechlorination catalyst Cat3 was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com