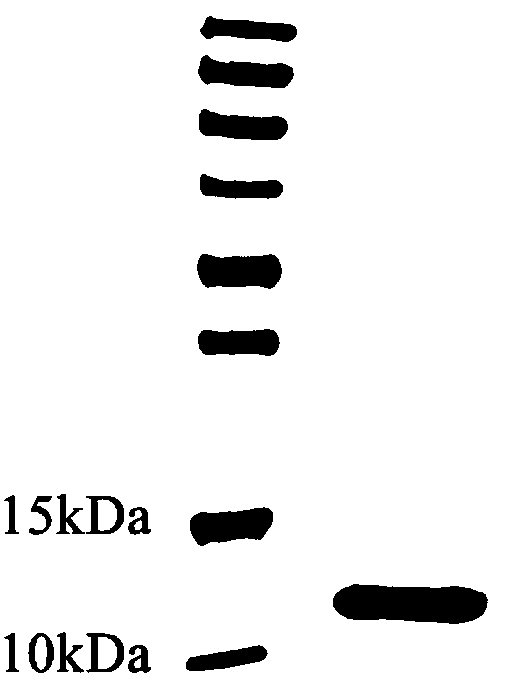Fusion protein HFBI-RGD gene and protein
A fusion protein and gene technology, applied in the field of protein genetic engineering, to achieve short production cycle, good solubility, and solve the effect of dispersion problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Construction of pET-28a-HFBI-RGD:
[0051] Using the HFBI nucleotide sequence shown in SEQ ID NO.3 in Trichoderma reesei as a template, using F1 as a forward primer, and R1 as a reverse primer, the first round of PCR was carried out to obtain the first PCR product; using the first PCR product as a template, F1 as a forward primer, and R2 as a reverse primer, the second round of PCR was performed to obtain the first sequence containing the fusion protein HFBI-RGD gene, which was digested and T4 DNA ligase ligation, the fusion protein HFBI-RGD gene was constructed on the PET-28a vector, and the PET-28a-HFBI-RGD plasmid was obtained. Specific steps are as follows:
[0052] a) enzyme digestion system
[0053]
[0054] b) conditions
[0055] i. Digest the target gene for 1 hour at 37°C.
[0056] ii. Digest the plasmid for 1 hour at 37°C.
[0057] iii. After digestion, inactivate at 80°C for 5 minutes.
[0058] c) Using the HFBI nucleotide sequence shown in...
Embodiment 2
[0071] Example 2 Construction of pPIC9k-HFBI-RGD:
[0072] 1) The vector is selected as pPIC9k, the restriction sites are NotI and EcoRI, and the primers are designed for PCR. The PCR system is shown in Table 3. Primers were designed as F2, R3. Using the plasmid PET-28a-HFBI-RGD constructed in Example 1 above as a template, PCR can obtain the sequence of the second fusion protein HFBI-RGD gene whose restriction sites are NotI and EcoRI.
[0073] 2) The PCR product and the vector pPIC9k were double-digested with restriction endonucleases NotI and EcoRI, respectively, so that the target gene fragment and the vector had cohesive ends. The specific enzyme digestion system and method are as follows:
[0074]
[0075] 3) Ligate the target gene fragment with cohesive ends and the vector pPIC9k using T4 DNA ligase, and the ligation system is shown in Table 6 above.
[0076] 4) After conversion and double enzyme digestion verification, the positive results obtained are verified b...
Embodiment 3
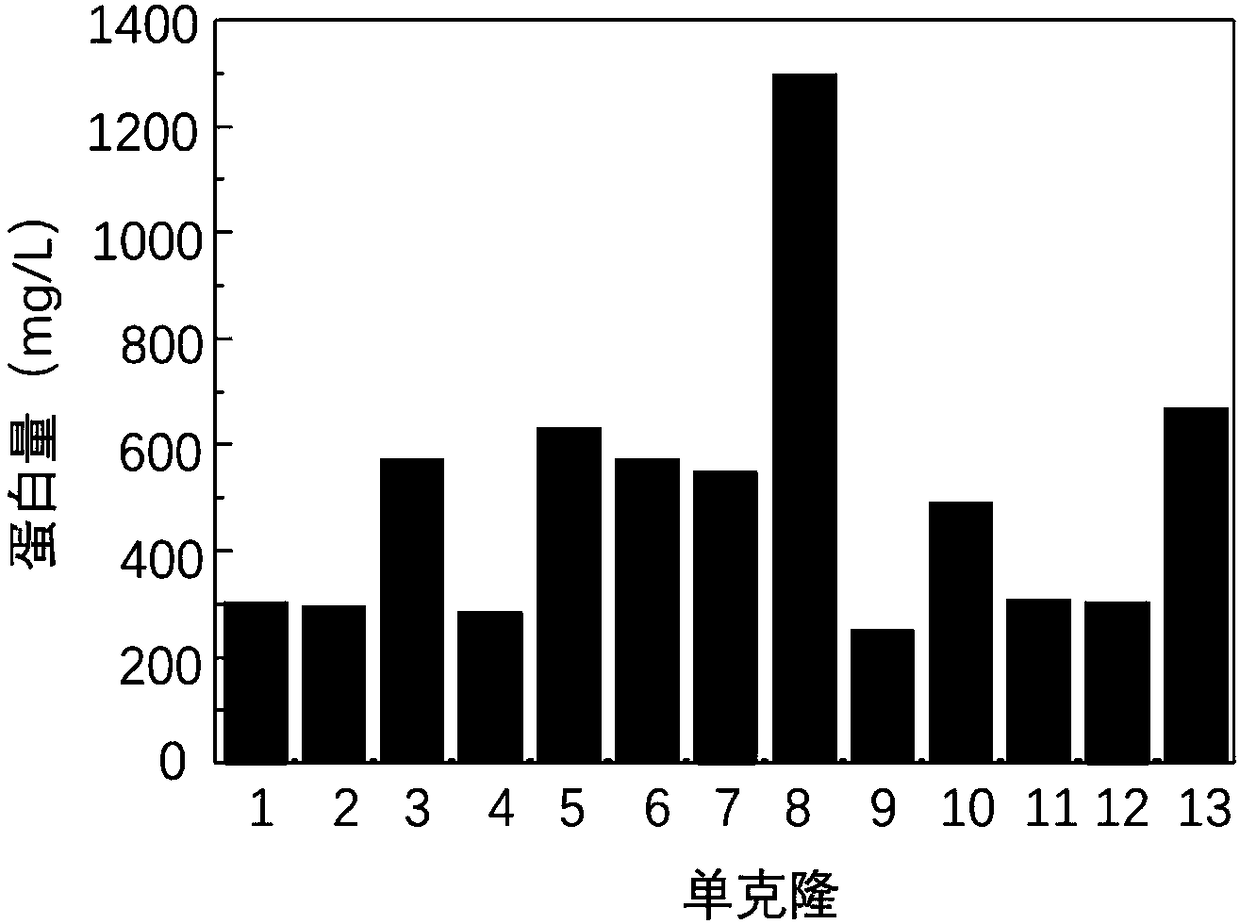
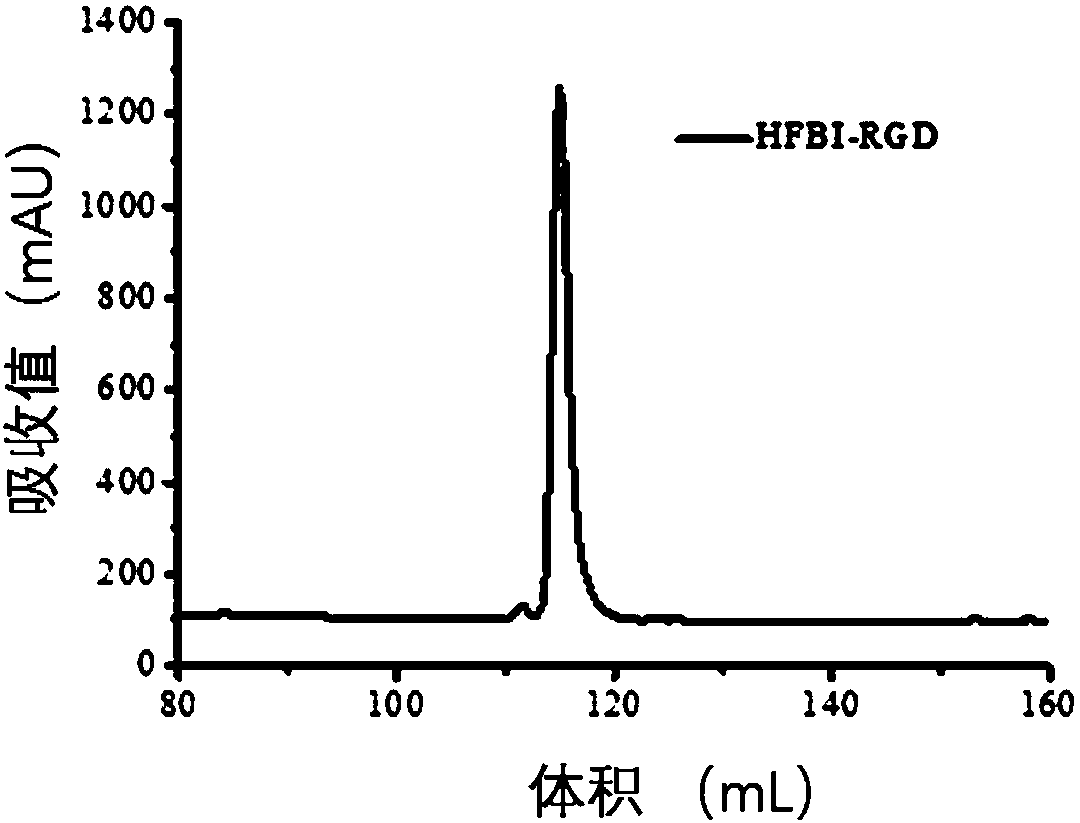
[0077] Example 3 Fusion protein HFBI-RGD positive clone screening and expression purification:
[0078] 1) For the recombinant expression vector to be inserted into the yeast genome at a higher copy in yeast cells, it needs to be linearized before being transferred into yeast cells. Using the restriction site analysis function provided in the primer design software Primer 5.0 to analyze the restriction sites of pPIC9k and HFBI-RGD genes, combined with the restriction site information provided by Ivitrogen in the Pichia expression system operation manual, the final The vector was digested and linearized with the restriction endonuclease SalI. The reaction system for enzyme digestion linearization is shown in Table 7. This system was digested in a water bath at 37°C for 2 hours. Take 5 μL of the product for agarose gel electrophoresis to check whether the digestion is complete, and then recover the band of the product by gel.
[0079] Table 7 Linearization system
[0080] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com