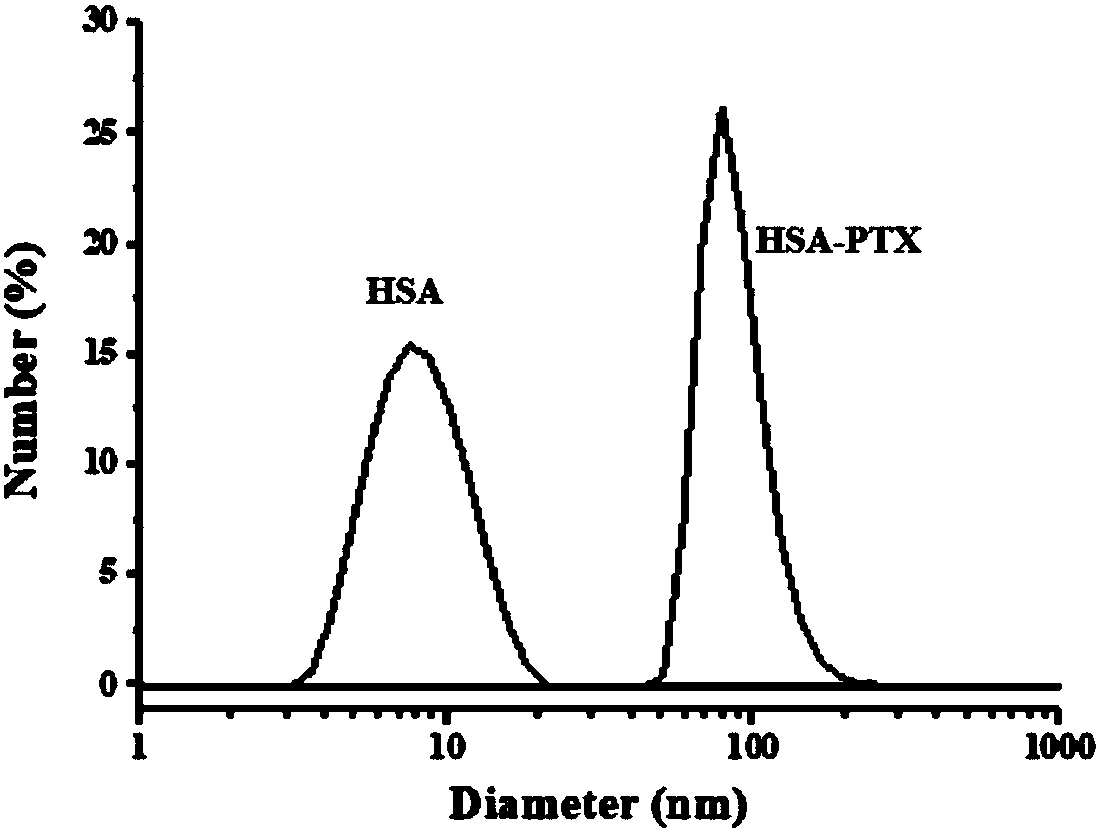
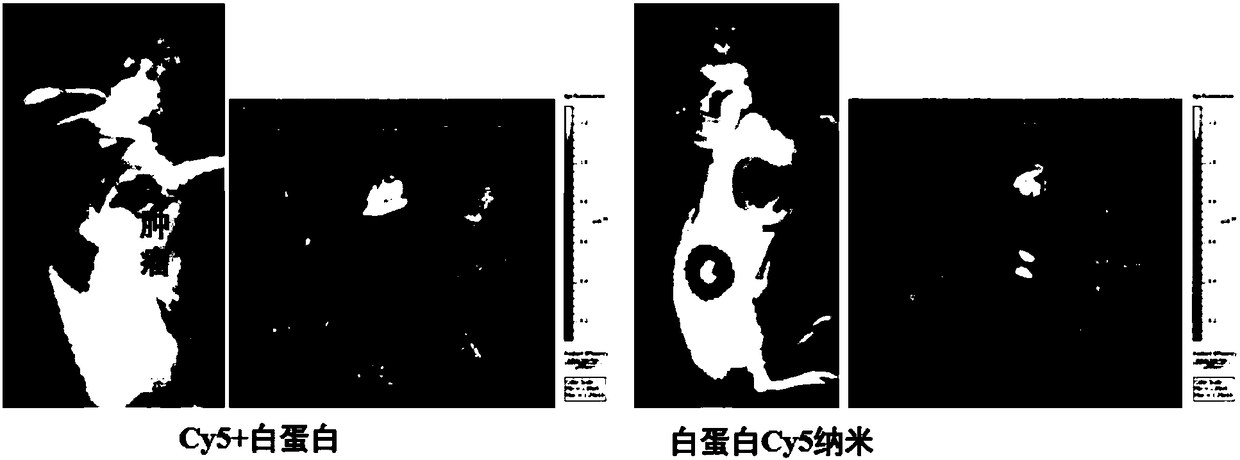
Albumin nanoparticles covering pharmacological active substances as well as preparation method and application thereof
A nanotechnology of albumin and pharmacological activity, which is applied in the direction of drug combination, pharmaceutical formula, organic active ingredients, etc., can solve the problems that have not yet been reported on oral drug delivery targeting system, and achieve improved solubility, complete biocompatibility, The effect of uniform size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of Example 1 Albumin Nanoparticles (HSA NP):
[0036] (1) Mix albumin solution in the range of 150mg / mL with 30mM glutathione solution (phosphate buffer solution of glutathione, pH value is 5.0-9.0), and stir for 100min at 20°C reaction to obtain a homogeneous solution of albumin with a final protein concentration of 50 mg / mL in which the spatial structure is developed;
[0037] (2) Add 10 mM sodium selenite solution to the albumin homogeneous solution with spatial structure unfolded to obtain a final concentration of protein in the albumin solution of 30 mg / mL, stir at 4 ° C for 6 h, and obtain a crude solution of albumin nanoparticles ;
[0038] (3) Put the crude solution of albumin nanoparticles into a dialysis bag, the molecular cut-off of dialysis is not less than 1000, and dialyze in a PBS solution at 0-20°C to remove excess glutathione, selenium compounds and their by-products to obtain Albumin nanoparticles.
[0039] The albumin nanoparticles were...
Embodiment 2
[0040] Preparation of Example 2 Albumin Nanoparticles (HSA NP):
[0041] (1) Mix albumin solution in the range of 100mg / mL with 20mM glutathione solution (phosphate buffer solution of glutathione, pH value is 5.0-9.0), and stir at 30°C for 20min reaction to obtain a homogeneous solution of albumin with a final protein concentration of 50 mg / mL in which the spatial structure is developed;
[0042] (2) Add 5mM sodium selenite solution to the albumin homogeneous solution developed by the spatial structure to obtain a final albumin concentration of 20 mg / mL in the albumin solution, stir at 10°C for 8h, and obtain crude albumin nanoparticles. solution;
[0043] (3) Put the crude solution of albumin nanoparticles into a dialysis bag, the molecular cut-off of dialysis is not less than 1000, and dialyze in a PBS solution at 0-20°C to remove excess glutathione, selenium compounds and their by-products to obtain Albumin nanoparticles.
[0044] The albumin nanoparticles were digested ...
Embodiment 3
[0045] Preparation of Example 3 Albumin Nanoparticles (HSA NP):
[0046] (1) Mix albumin solution in the range of 200mg / mL with 5mM glutathione solution (phosphate buffer solution of glutathione, pH value is 5.0-9.0), and stir for 60min at 30°C reaction to obtain a homogeneous albumin solution with a final protein concentration of 80 mg / mL in which the spatial structure is expanded;
[0047] (2) Add 15 mM sodium selenite solution to the albumin homogeneous solution with spatial structure unfolded to obtain a final albumin concentration of 60 mg / mL in the albumin solution, and stir at 10 ° C for 8 hours to obtain crude albumin nanoparticles. solution;
[0048] (3) Put the crude solution of albumin nanoparticles into a dialysis bag, the molecular cut-off of dialysis is not less than 1000, and dialyze in a PBS solution at 0-20°C to remove excess glutathione, selenium compounds and their by-products to obtain Albumin nanoparticles.
[0049] The albumin nanoparticles were digest...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size distribution | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com