Deer-derived bovine viral diarrhea inactivated vaccine and preparation method thereof
A technology of bovine viral diarrhea and inactivated vaccine, applied in the field of vaccine, can solve the problems of unsatisfactory immune effect of sika deer mucosal disease, miscarriage, stillbirth, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Isolation and acquisition of deer-derived bovine viral diarrhea virus JL05 strain
[0026] The deer-derived bovine viral diarrhea virus (BVDV) JL05 strain used in the present invention is isolated from the spleen of suspected diseased sika deer. Inoculation of the isolated virus into MDBK cells can produce obvious cytopathic changes, which were detected by neutralization test and indirect immunofluorescence test using BVDV positive antibody; according to the full gene sequence of BVDV published by GenBank, specific primers were designed and synthesized as follows:
[0027] BU: 5'-TCGACGCTTTGGAGGACA-3';
[0028] BD: 5'-CCATGTGCCATGTACAG-3'.
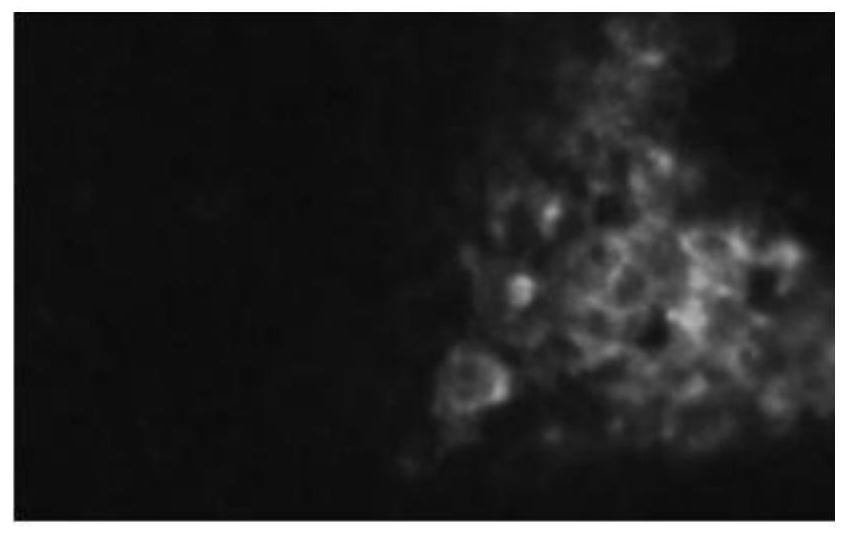
[0029] The size of the target fragment is 186bp, which is used for RT-PCR detection of BVDV. The results showed that the isolated virus was BVDV, and the results of fluorescence identification were as follows: figure 1 As shown, the PCR identification results are as follows figure 2 shown.
[0030] According to the w...
Embodiment 2
[0033] Example 2 Passage and Plaque Cloning Purification of Deer-derived BVDV JL05 Strain
[0034] The isolated deer-derived BVDV JL05 strain was continuously passaged in vitro through MDBK cells to 30 passages. Plaque cloning and purifying viruses were carried out at intervals of 5 generations (ie F5, F10, F15, F20, and F25 generations were carried out for plaque cloning and purifying viruses). Take the deer-derived BVDVJL05 strain virus for 10-fold serial dilution, and the dilution range is 10 -2 ~10 -6 After the virus is diluted, inoculate a 6-well cell culture plate full of 80% monolayer MDBK cells, 1ml / well, discard the virus solution after 1 hour of adsorption, wash 3 times with serum-free DMEM, add 2×DMEM and sterilized agar solution Mixed solution, 3ml / well, cooled at room temperature and placed at 37°C, 5% CO 2 Cultivate in an incubator for 3-4 days, observe the plaques, pick a single plaque in a well with few plaques, save it and pass it down.
Embodiment 3
[0035] The preparation of embodiment 3 seedlings with antigen solution
[0036] 1. Preparation of deer-derived BVDV JL05 strain antigen by adherent culture cells in spinner bottles
[0037]Cultivate MDBK cells in spinner bottles. When the cells are 90-100% monolayer, they are digested and passaged with trypsin-EDTA cell dispersion solution, dispersed into new spinner bottles at a ratio of 1:3-1:5, and the cells grow The solution is DMEM containing 5-8% newborn bovine serum. When the cells grew to 80-90% monolayer, the deer-derived BVDV JL05 strain of the present invention was inoculated into MDBK cells with an infectious dose (MOI) of 0.01-0.05, and the cell maintenance solution was DMEM containing 3% horse serum, and continued After culturing for 60-84 hours, the virus liquid was harvested when the cytopathic effect reached 80-90%, and stored at -20°C for later use.
[0038] 2. Preparation of deer-derived BVDV JL05 strain antigen using microcarrier suspension culture techno...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com