Preparation method of phospholene oxide, substituted phospholene oxide and preparation method of substituted phospholene oxide
A technology for oxides and cyclophosphene, which is applied in the field of preparation of cyclophosphene oxides, can solve the problems of low yield of small group substitutions, high price, limited application and the like, and achieves simple and feasible methods, low cost, and products. high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
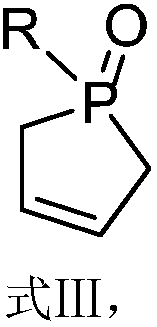
[0057] The preparation route of the methyl-substituted cyclophosphene oxide of this embodiment is as follows, and the specific preparation method includes the following steps:
[0058]
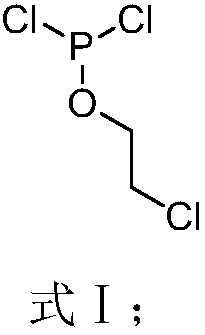
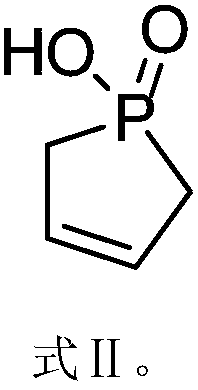
[0059] 1) Preparation of cyclophosphene oxide
[0060] Under ice-bath conditions, add 250 mL of methylene chloride and 137.0 g of phosphorus trichloride into a reactor equipped with a thermometer, agitator and tail gas absorption device, and add 410.0 g of sodium hydroxide with a mass fraction of 5% in the tail gas absorption device Aqueous solution, then add 41.1g of chloroethanol dropwise to the reactor while stirring, keep the temperature below 0°C during the dropwise addition of chloroethanol, then raise the temperature to 20°C, keep the temperature at 20°C for 1 hour, and remove the solvent by distillation The product was purified to obtain 72.9g of compound 1a;
[0061] Add 72.9g of compound 1a, 0.6g of copper stearate, 1.4g of ferric chloride, and 0.8g of BHT to the autoclave under ...
Embodiment 2
[0065] The preparation method of the methyl-substituted cyclophosphene oxide of the present embodiment comprises the following steps:
[0066]
[0067] 1) Preparation of cyclophosphene oxide
[0068] Under ice-bath conditions, add 250 mL of methylene chloride and 136.9 g of phosphorus trichloride into a reactor equipped with a thermometer, agitator and tail gas absorption device, and add 370.0 g of sodium hydroxide with a mass fraction of 10% in the tail gas absorption device Aqueous solution, then add 80.5g of chloroethanol dropwise to the reactor while stirring, during the process of dropwise adding chloroethanol, keep the temperature below 0°C, then raise the temperature to 30°C, keep the reaction at 30°C for 1.5 hours, remove the solvent by distillation The product was purified to obtain compound 1a144.3g;
[0069] Add 144.3g of compound 1a, 7.5g of copper stearate, 34.9g of ferric chloride, and 35.0g of BHT to the autoclave under an argon atmosphere, cool down to -20°...
Embodiment 3
[0073] The preparation method of the methyl-substituted cyclophosphene oxide of the present embodiment comprises the following steps:
[0074]
[0075] 1) Preparation of cyclophosphene oxide
[0076] Under ice-bath conditions, add 35 mL of methylene chloride, 27.4 g of phosphorus trichloride into the reactor with thermometer, stirrer and tail gas absorption device, and add 46.5 g of sodium hydroxide with a mass fraction of 15% in the tail gas absorption device Aqueous solution, then add 16.4g of chloroethanol dropwise to the reactor while stirring, during the process of dropping chloroethanol, keep the temperature below 0°C, then raise the temperature to 40°C, keep it at 40°C for 0.5 hours, and remove the solvent by distillation The product was purified to obtain 27.5 g of compound 1a;
[0077] Add 27.5g of compound 1a, 2.7g of copper stearate, 5.5g of ferric chloride, and 2.7g of BHT into the autoclave under an argon atmosphere, drop the temperature to -30°C and feed 68.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com