A temperature-sensitive injectable lacrimal plug and its preparation method
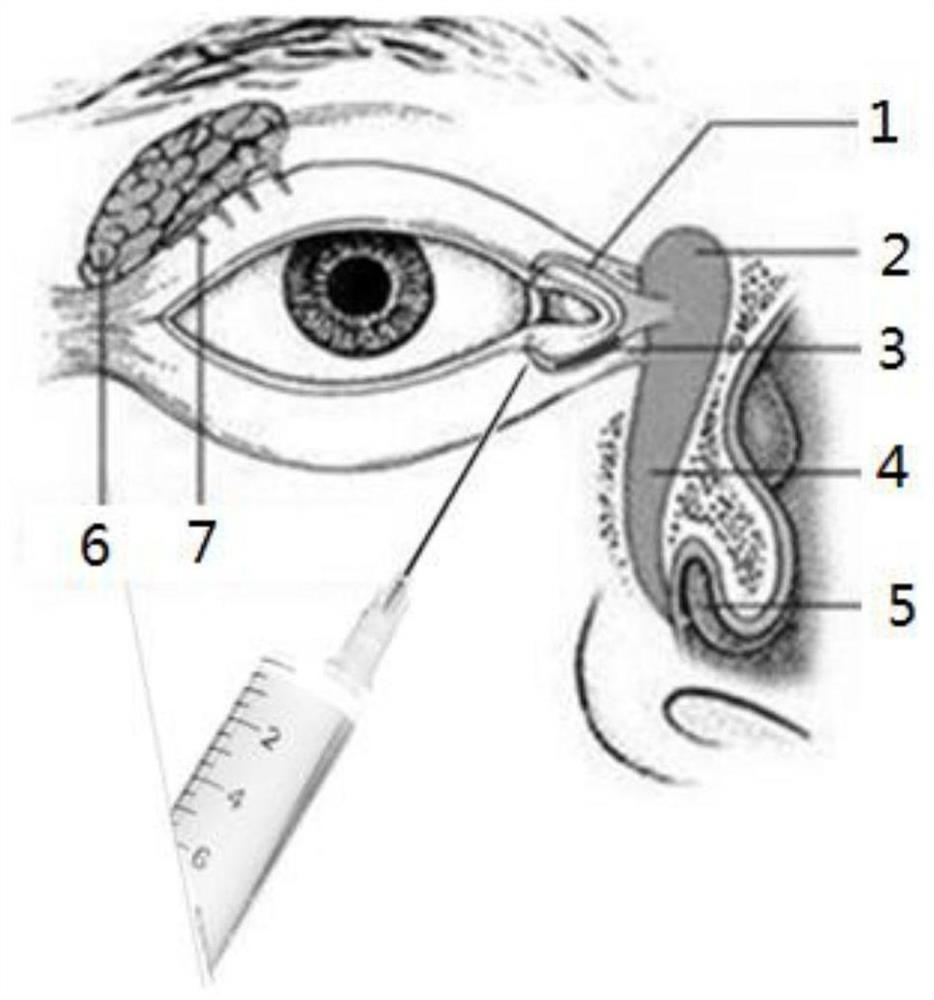
A lacrimal duct suppository and injection-type technology, which can be used in pharmaceutical formulations, surgery, drug delivery, etc., can solve the problems of inconvenient implantation and removal of patients' eyes, less description of lacrimal duct suppository preparation techniques, and difficulty in popularization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]
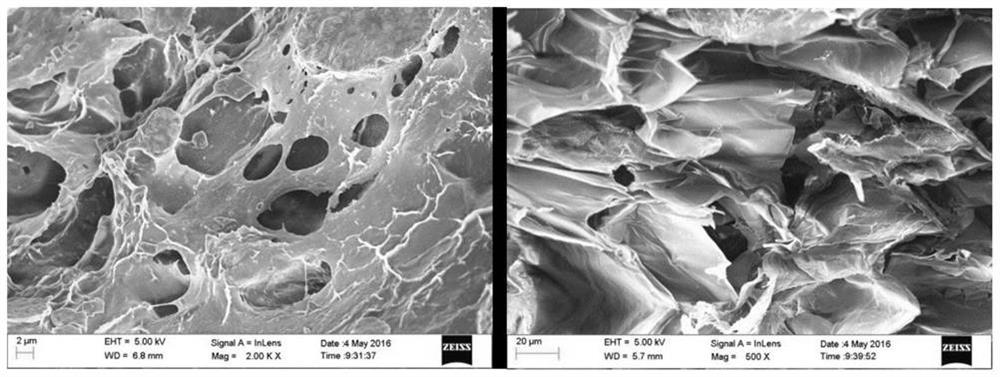
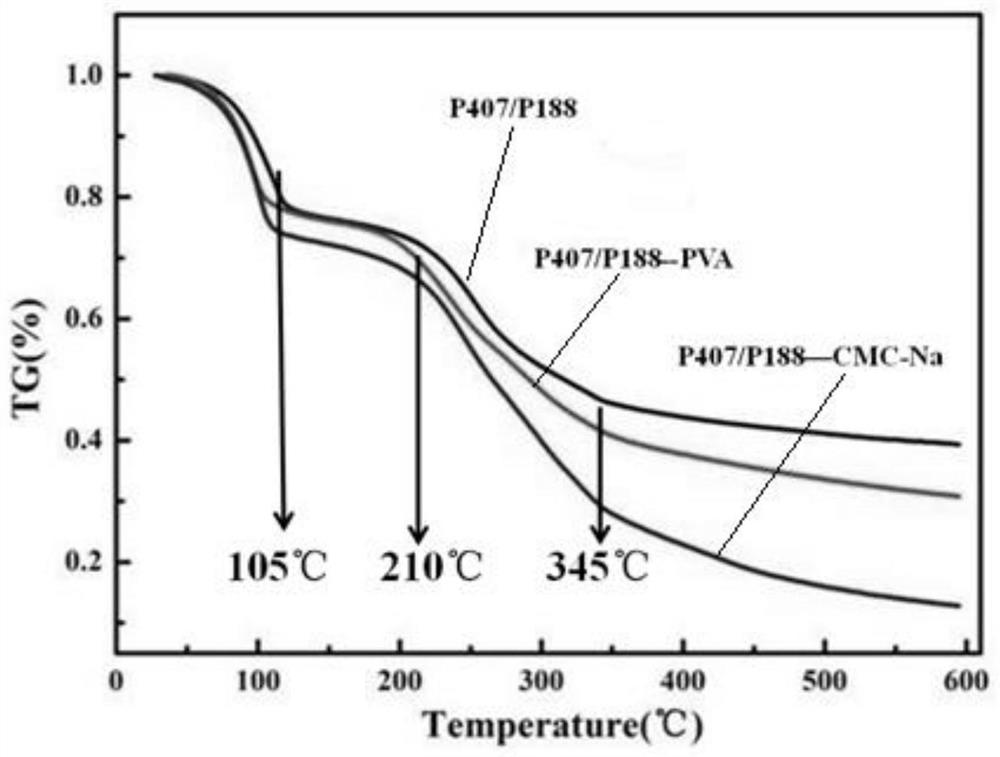
[0039] Test results: The solidification temperature of the product is 31°C; in the liquid state of 15°C, the viscosity of the ingredients is about 8mPa·s measured by a viscometer; under the curing condition of 31°C, a columnar product with a diameter of 3mm and a height of 4mm is prepared, measured Obtained its extrusion strength is 15Pa. The results show that the material can basically meet the functional requirements of injectable lacrimal plugs, but the viscosity is low in liquid state and the mechanical strength is low after curing.
Embodiment 2
[0041]
[0042]
[0043] Test results: The solidification temperature of the product is 31°C; in the liquid state of 15°C, the viscosity of the ingredients is about 70mPa·s measured by a viscometer; under the curing condition of 31°C, a columnar product with a diameter of 3mm and a height of 4mm is prepared, measured Obtained its extrusion strength is 15Pa. The results show that the material can basically meet the functional requirements of injectable lacrimal plugs, but the mechanical strength is low after curing.
Embodiment 3
[0045]
[0046] Test results: The solidification temperature of the product is 31°C; in the liquid state of 15°C, the viscosity of the ingredients measured by a viscometer is about 8.1mPa·s; under the curing condition of 31°C, a columnar product with a diameter of 3mm and a height of 4mm is prepared. Its extrusion strength was measured to be 0.50 MPa. The results show that the material can basically meet the functional requirements of injectable lacrimal plug, but the viscosity is low in liquid state.
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com