Method for preparing pneumocandin B0 by microbial fermentation
A technology of neumocontin and fermentation process, applied in the field of fermentation, can solve the problems of lack of large-scale, high-purity and stable production of nemocontin B, affecting the fermentation purity and yield of nemocontin B, etc. pressure, stable production effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: Neomocontin B 0 5L fermentation production
[0024] Prepare the primary shake flask seed medium, the medium components are as follows: anhydrous glucose 4%, soybean cake powder 2%, cottonseed cake powder 1%, corn steep liquor dry powder 1%, potassium dihydrogen phosphate 0.1%, trace element source solution (magnesium chloride hexahydrate 0.486g / L, zinc sulfate heptahydrate 0.418g / L, ferric chloride hexahydrate 0.840g / L, copper sulfate pentahydrate 0.078g / L) 0.1%. Prepare drinking water and adjust the pH to 5.5 before sterilization. Use a sterilizer to sterilize at 121°C for 30 minutes.
[0025] First-level shake flask seed culture: Inoculate 1ml of the seed glycerol tube into the first-level seed shake flask, place on a shaker at 25°C, 220rpm, and cultivate for 3 days.
[0026] Prepare the secondary shake flask seed medium, the medium components are as follows: anhydrous glucose 4%, soybean cake powder 2%, cottonseed cake powder 1%, corn steep liquor dr...
Embodiment 2-7
[0030] Embodiment 2-7: Different fermentation media produce pneumocidine B 0
[0031] Using fermentation media composed of different carbon sources, according to the fermentation method of Example 1, Nemocontin B was prepared 0 . The inorganic salt components in the fermentation medium are: 0.4% of dipotassium hydrogen phosphate, 0.05% of ferrous sulfate, 0.03% of manganese sulfate, 0.2% of ammonium sulfate and 0.2% of sodium nitrate. The carbon source and nitrogen source components of the fermentation medium are shown in the table below.
[0032] Example
Embodiment 8
[0034] Sampling during the fermentation process for the determination of pneumocidine B in the fermentation broth 0 and main impurity pneumocontin A 0 , serine analogs, D 0 The processing process is as follows: take a small amount of fermentation liquid, add ethanol, ultrasonic for 30 minutes, centrifuge to get the supernatant, and measure its yield by HPLC.
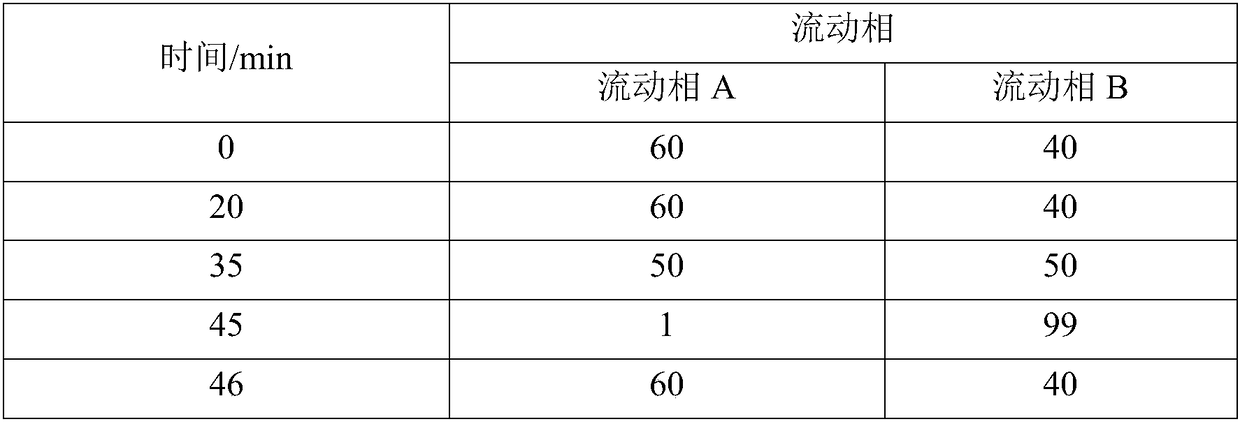
[0035] HPLC detection conditions: YMC J'sphere ODS-M80 4μm 250×4.6mm chromatographic column; A: 0.1% phosphoric acid aqueous solution B: acetonitrile as mobile phase, gradient elution; detection wavelength is 210nm; flow rate is 1.5mL / min; The column temperature was 30°C. The elution gradient is shown in the table below:
[0036]
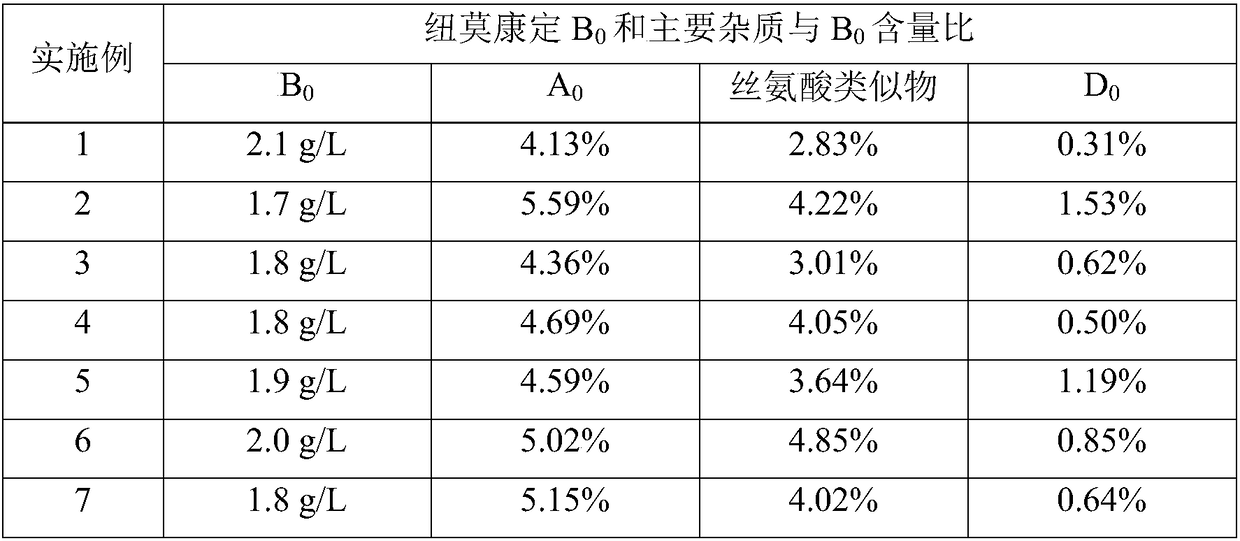
[0037] Adopt above-mentioned method to measure the pneumocantine B of the fermented liquid gained in embodiment 1-7 0 And the main impurity content, see the table below.
[0038]
[0039] It can be seen from the above table that when the carbon source of the fermentation medium is a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com