Ratio fluorescence probe for detecting hydroxylamine as well as synthesis method and application thereof
A technology of fluorescent probes and synthesis methods, applied in the field of organic small molecule fluorescent probes, can solve the problems of lack of fluorescent probes, and achieve the effects of easy-to-obtain raw materials, simple and easy synthesis process, and high specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Synthesis of Fluorescent Probes
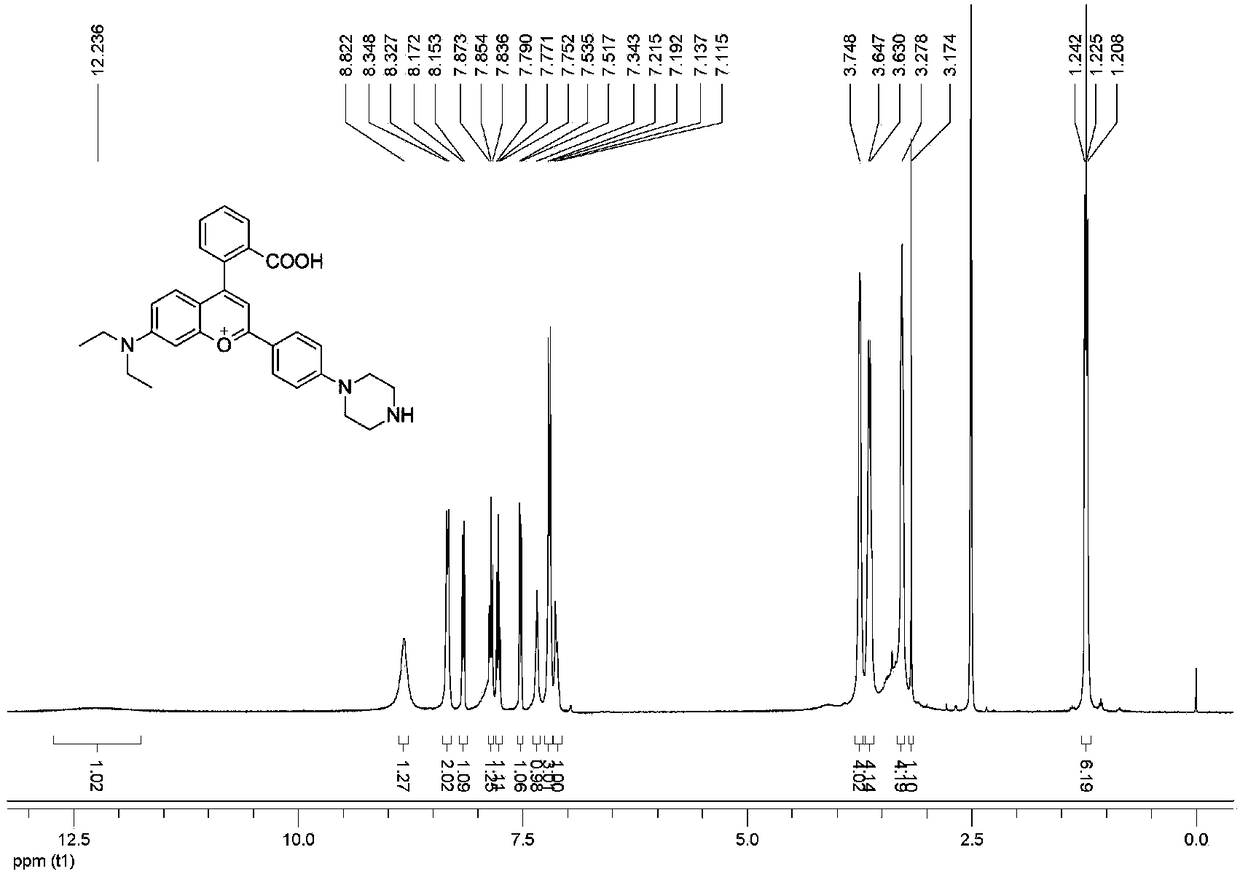
[0033] Add raw material 1 (1 mmol) and raw material 2 (1 mmol) into 5 mL of concentrated sulfuric acid, heat the reaction at 90 °C for 4 hours, and cool to room temperature. The reaction solution was poured into 10 mL of ice water, and 2 mL of perchloric acid was slowly added dropwise to precipitate a red solid, which was separated and purified to obtain the pure compound 1 (yield 52%) of the fluorescent probe. 1 H NMR spectrum see figure 1 .
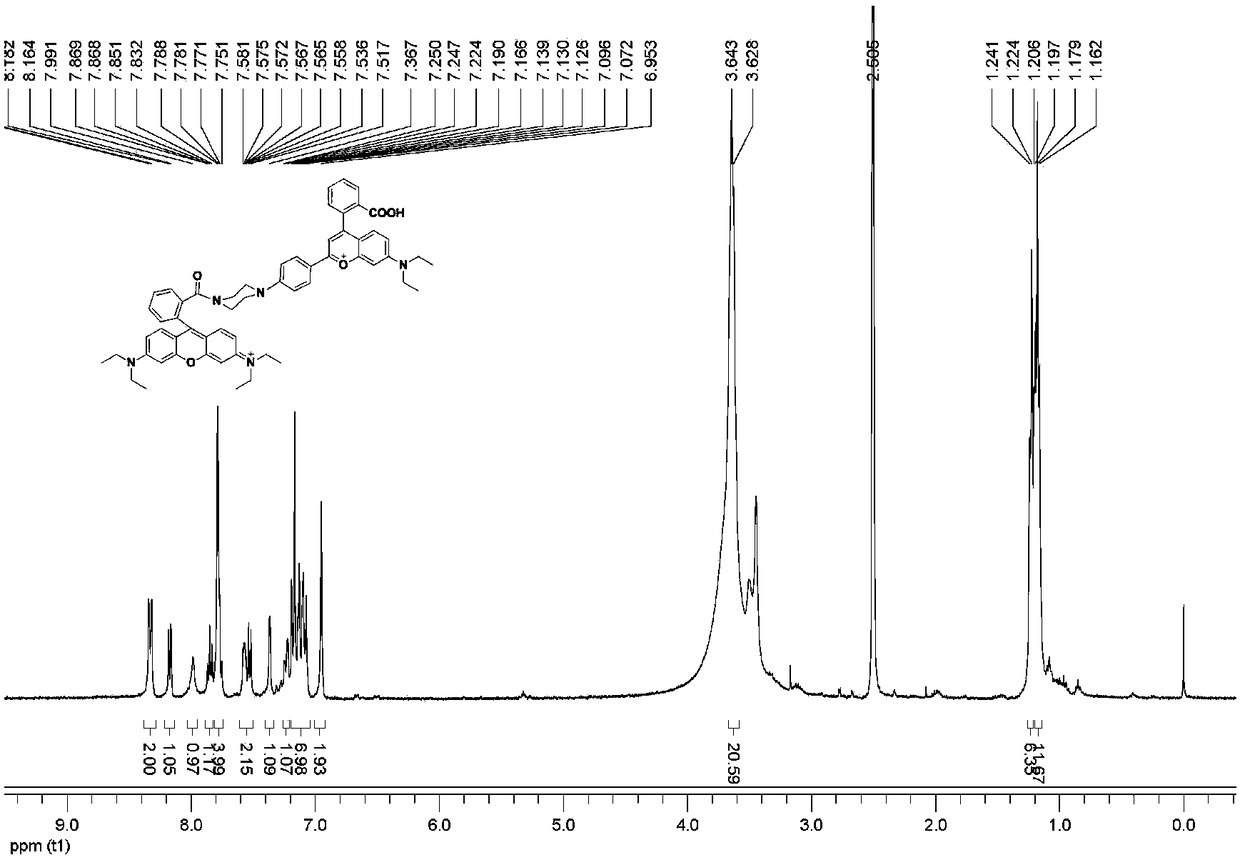
[0034] Dissolve Rhodamine B (1 mmol) and 1 mL of phosphorus oxychloride in 15 mL of dichloroethane and heat to reflux. After reacting for 3 h, spin to remove dichloroethane, and add 15 mL of dichloromethane to dissolve. Subsequently, the solution was slowly added dropwise to a dichloromethane solution of compound 1 (containing 0.2 mL triethylamine) at 0 °C, and stirred at 25 °C for 4 h. After the reaction, the reaction solution was spin-dried, and the pure fluorescent probe (yield 2...
Embodiment 2
[0035] The response of embodiment 2 fluorescent probes to hydroxylamine
[0036] Dissolve the probe compound obtained in Example 1 with ethanol, add PBS buffer to prepare a 5 μM probe buffer solution (containing 20% ethanol, pH = 7.4), which is the probe working solution.
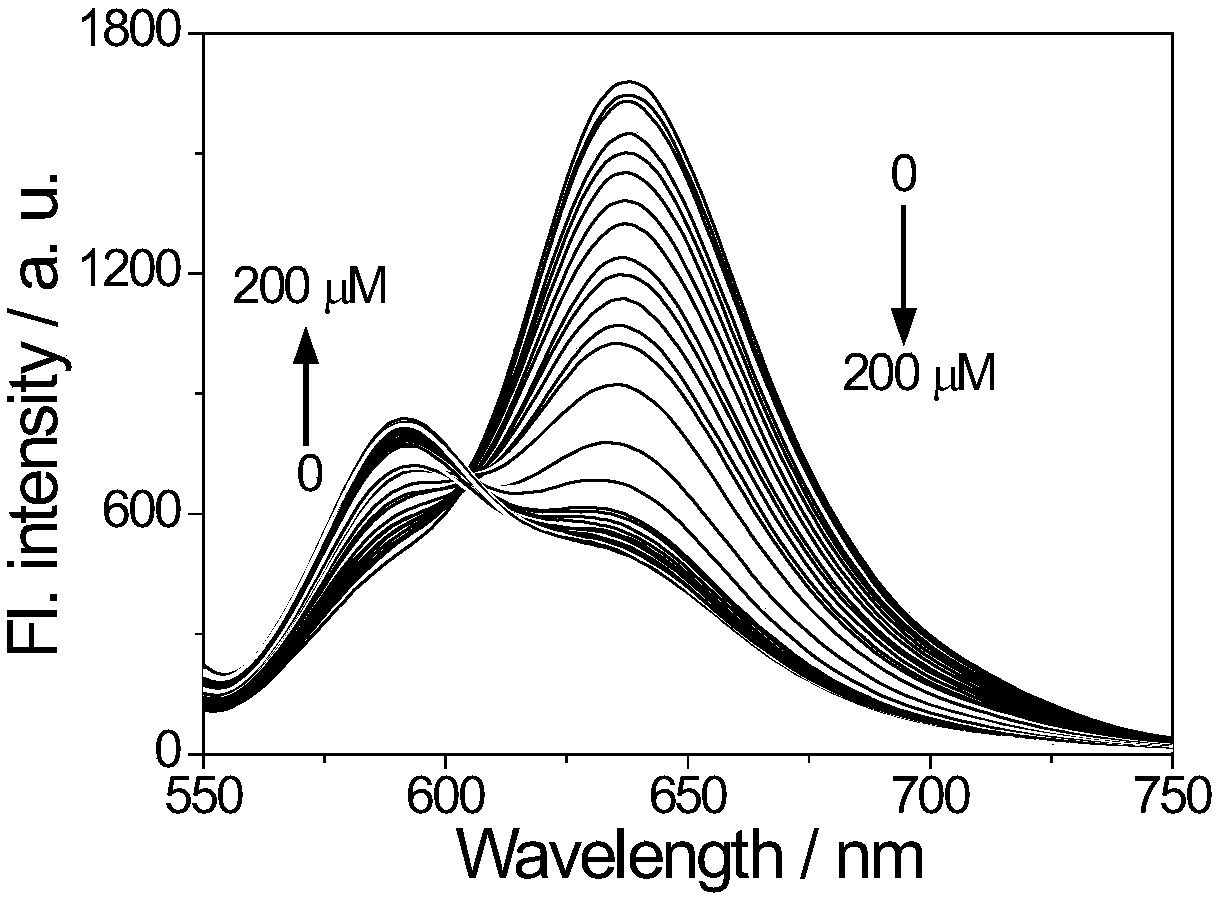
[0037] Hydroxylamine was supplied in aqueous solution with hydroxylamine hydrochloride; added to the above needle buffer solution to increase the concentration of hydroxylamine from 5 μM to 200 μM, a total of 40, adding an equal amount of buffer as a control; then perform fluorescence detection (λ Ex = 540nm), calculate the fluorescence intensity in each system; by analyzing the relationship between the ratio of the fluorescence intensity at 590 nm and 637nm and the concentration of hydroxylamine, evaluate the response performance of the probe to hydroxylamine (see image 3 and Figure 4 ). figure 2 It shows that with the increase of hydroxylamine concentration, the fluorescence intensity at 590 nm of ...
Embodiment 3
[0038] Embodiment 3 Fluorescent probe specificity analysis to hydroxylamine
[0039] Take 5 mL of the probe working solution in Example 2, and then add 50 μL of probe, Hcys, Na 2 S, Na 2 SO 3 , Cys, GSH, Vc, Zn 2+ , Fe 2+ , Fe 3+ , Mg 2+ , arginine, ammonia water, ammonium chloride and other related analytes, the numbers are 1-14 in sequence, and the number 15 is hydroxylamine. Fluorescence detection is then performed (λ Ex = 540nm), calculate the fluorescence intensity in each system, and evaluate the interference of the different substances on the fluorescent probe solution (see Figure 5 ). It can be seen from the figure that when Hcys and Na are added to the probe solution 2 S, Na 2 SO 3 , Cys, GSH, VC, Zn 2+ , Fe 2+ , Fe 3+ , Mg 2+ , arginine, ammonia water, ammonium chloride and other related analytes, only hydroxylamine can cause significant fluorescence changes in the solution, while the fluorescence of the solution does not change when other small molec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com