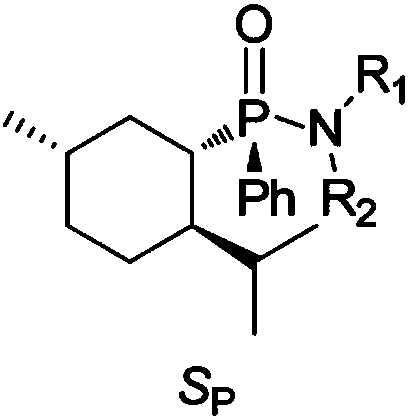
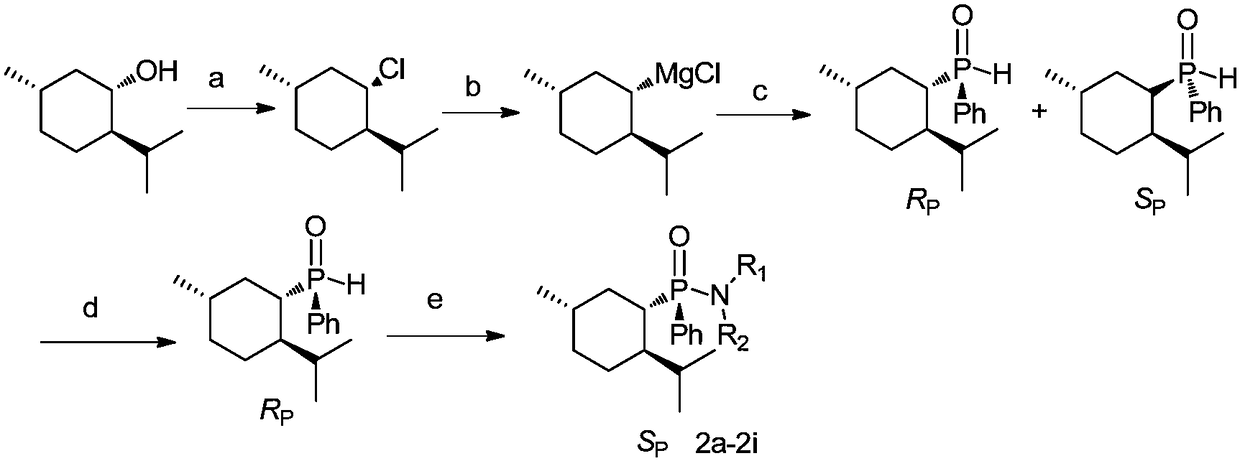
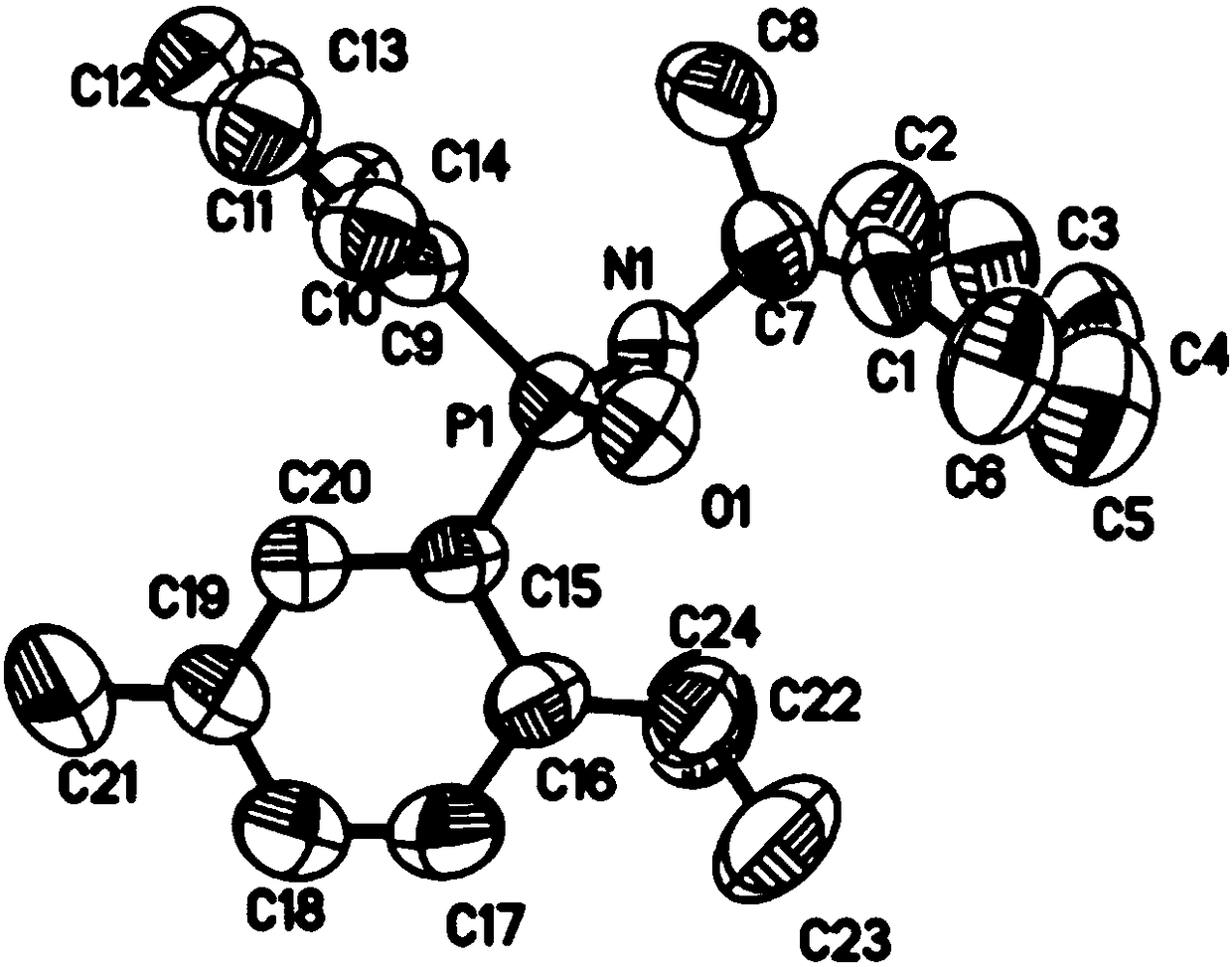
Chiral menthyl phenyl phosphonamide compound and preparation method thereof
A technology for charged phenylphosphonamide and compound, which is applied in the field of chiral menthyl phenylphosphonamide compounds and preparation, and achieves the effect of simple and easy-to-obtain method and obvious chiral induction effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] A chiral menthyl phenylphosphonamide compound 2a, comprising the following structural formula:
[0064]
[0065]A preparation method of chiral menthyl phenylphosphonamide compound 2a, comprising the steps of:
[0066] Preparation of pure R-menthylphenylphosphine hydrogen
[0067] a. Add menthol 0.17mol to 37% zinc chloride hydrochloric acid solution, stir at 35°C for 5 hours, return to room temperature, extract with 50mL of n-hexane, wash the organic phase with 30mL of water, and then wash with 10mL of concentrated sulfuric acid, water Wash with 30 mL, take the organic phase, dry with anhydrous magnesium sulfate, and remove the organic phase under reduced pressure to obtain a colorless oil;
[0068] b. Add 0.1 mol of magnesium, 0.1 mol of menthyl chloride and 50 mL of tetrahydrofuran in sequence in the three-necked flask to react for 6 hours to obtain the menthyl Grignard reagent;
[0069] c. Under the condition of an ice-water bath, add the prepared menthyl Grigna...
Embodiment 2
[0074] A chiral menthylphenylphosphonamide compound 2b, comprising the following structural formula:
[0075]
[0076] A preparation method of chiral menthyl phenylphosphonamide compound 2b, comprising the steps of:
[0077] The preparation of pure R-menthylphenylphosphine hydrogen is the same as in Example 1.
[0078] Preparation of Chiral Menthyl-Phenylphosphonamide Compound 2b
[0079] Add R-menthylphenylphosphine hydrogen (52.8mg, 0.2mmol), triethylamine (0.4mmol, 0.06mL) and carbon tetrachloride (2mmol) successively in the reaction flask, then add solvent acetonitrile (1mL), in n-Propylamine (0.2 mmol) was added at 0°C. The reaction was stirred for 30 minutes. Then warm to room temperature and stir overnight. Treat the reaction, add water (5mL) and ethyl acetate (5mL×3) to extract three times, dry over anhydrous magnesium sulfate, and vacuumize to obtain a crude product, then add petroleum ether (1mL) and dichloromethane (0.2mL) to weigh Crystallized to obtain 58....
Embodiment 3
[0081] A chiral menthyl phenylphosphonamide compound 2c, comprising the following structural formula:
[0082]
[0083] A preparation method of chiral menthyl phenylphosphonamide compound 2c, comprising the steps of:
[0084] The preparation of pure R-menthylphenylphosphine hydrogen is the same as in Example 1.
[0085] Preparation of chiral menthylphenylphosphonamides 2c
[0086] Add R-menthylphenylphosphine hydrogen (52.8mg, 0.2mmol), triethylamine (0.4mmol, 0.06mL) and carbon tetrachloride (2mmol) successively in the reaction flask, then add solvent acetonitrile (1mL), in n-Butylamine (0.2 mmol) was added at 0°C. The reaction was stirred for 30 minutes. Then warm to room temperature and stir overnight. Treat the reaction, add water (5mL) and ethyl acetate (5mL×3) to extract three times, dry over anhydrous magnesium sulfate, and vacuumize to obtain a crude product, then add petroleum ether (1mL) and dichloromethane (0.2mL) to weigh Crystallized to obtain 61.1 mg of p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com