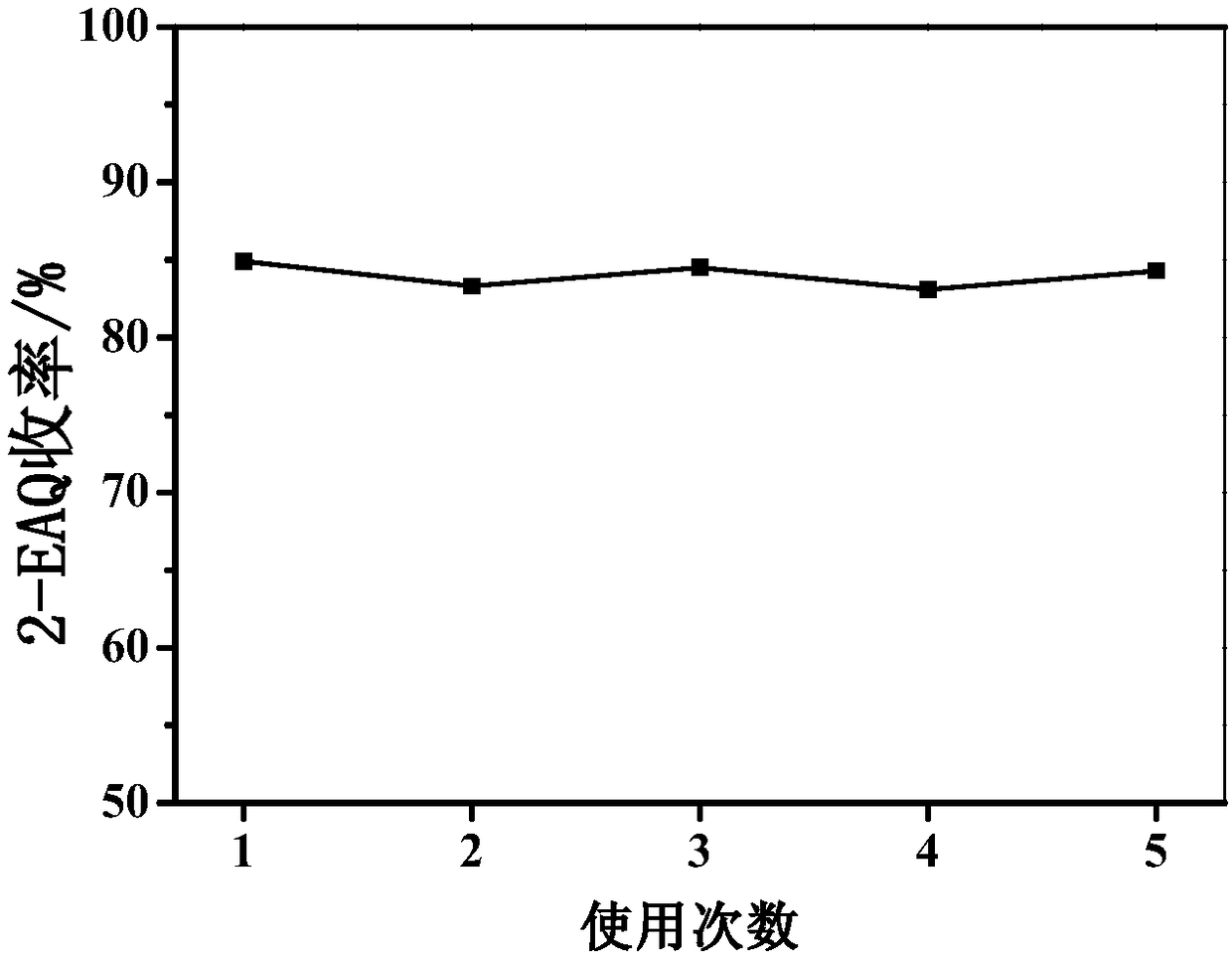
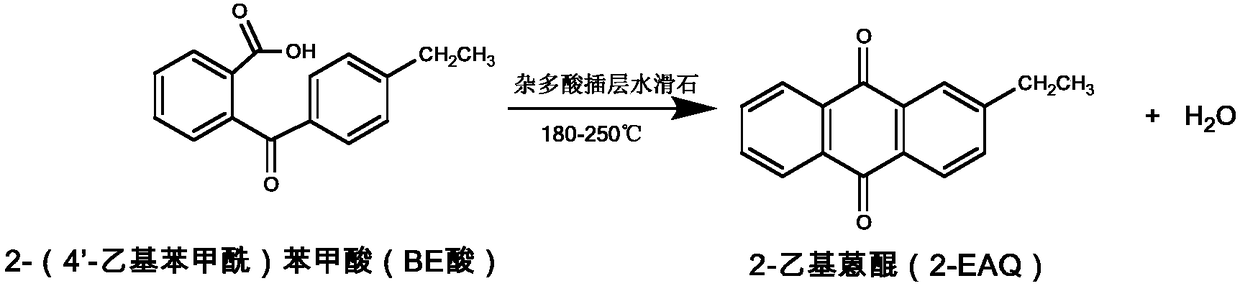
Method for synthesizing 2-ethylanthraquinone by taking heteropoly acid intercalated hydrotalcite as catalyst
A technology of ethyl anthraquinone and heteropoly acid, applied in the field of preparation of 2-ethyl anthraquinone, can solve equipment and environmental hazards, the production scale and product quality cannot be satisfied, and restrict the industrial production scale of 2-ethyl anthraquinone Normal development and other problems, to achieve the effect of improving separation efficiency and less dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Catalyst preparation: prepare 15mmol MgCl 2 and 7.5mmol AlCl 3 75 mL of the mixed salt solution, and then prepare 0.5M 75ml tris solution, add the tris solution into the mixed salt solution, mix and stir for 0.5 hours, and conduct a hydrothermal reaction at 80°C for 12 hours. Gel material was obtained by centrifugation, in 0.5M Na 2 CO 3 dispersed in the solution, ion-exchanged for 1 hour, centrifuged and washed, and vacuum-dried to obtain magnesium-aluminum carbonate hydrotalcite for future use. Prepare 100mL of phosphotungstic acid sodium salt solution with a concentration of 1.1mM, add 2.3g of magnesium aluminum carbonate hydrotalcite to it, stir for 12 hours under the protection of no nitrogen, centrifuge, wash, and vacuum dry to obtain phosphotungstic acid intercalated trihydroxy Methyl hydrotalcite, the chemical formula is Mg 9 Al 3 (OH) 15 (C 4 h 8 NO 3 )(PW 12 o 40 ).
[0024] A. Catalytic synthesis of 2-ethylanthraquinone: Add 5.0g of 2-(4'-ethylben...
Embodiment 2
[0027]Catalyst preparation: change phosphotungstic acid sodium salt solution to silicotungstic acid sodium salt solution, and other conditions are the same as the catalyst preparation in Example 1 to obtain silicotungstic acid intercalated trimethylol hydrotalcite, the chemical formula is Mg 9 al 3 (OH) 14 (C 4 h 8 NO 3 )(SiW 12 o 40 ).
[0028] A. Catalytic synthesis of 2-ethylanthraquinone: Add 5.0g 2-(4′-ethylbenzoyl)benzoic acid into the reactor, add 50mL chloroform to fully dissolve, add 7.0g silicotungstic acid to intercalate trihydroxy Methyl hydrotalcite catalyst, after stirring for 15 minutes, the temperature was raised to 70°C to dechloroform, and the temperature was raised to 130°C to make the BE acid into a molten state, so that the molten BE acid was evenly adsorbed on the surface and layer of the catalyst. Then the temperature was raised to 180° C., and the reaction was carried out for 2.0 hours. Cool to room temperature, add 50mL of toluene to fully diss...
Embodiment 3
[0031] Catalyst preparation: change phosphotungstic acid sodium salt solution to phosphomolybdic acid sodium salt solution, and other conditions are the same as the catalyst preparation in Example 1 to obtain phosphomolybdic acid intercalated trimethylol hydrotalcite, the chemical formula is Mg 9 al 3 (OH) 15 (C 4 h 8 NO 3 ) (PMo 12 o 40 ).
[0032] A. Catalytic synthesis of 2-ethylanthraquinone: Add 5.0g 2-(4'-ethylbenzoyl)benzoic acid into the reactor, add 50mL chloroform to fully dissolve, add 5.0g phosphomolybdic acid to intercalate trihydroxy Methyl hydrotalcite catalyst, after stirring for 15 minutes, heat up to 70°C to remove the solvent chloroform, then heat up to 130°C to make the BE acid into a molten state, so that the molten BE acid is evenly adsorbed on the surface and layer of the catalyst, then heat up to 180°C, and react 2.0 hours. Cool to room temperature, add 50mL of toluene to fully dissolve the product, separate by filtration to obtain catalyst and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com