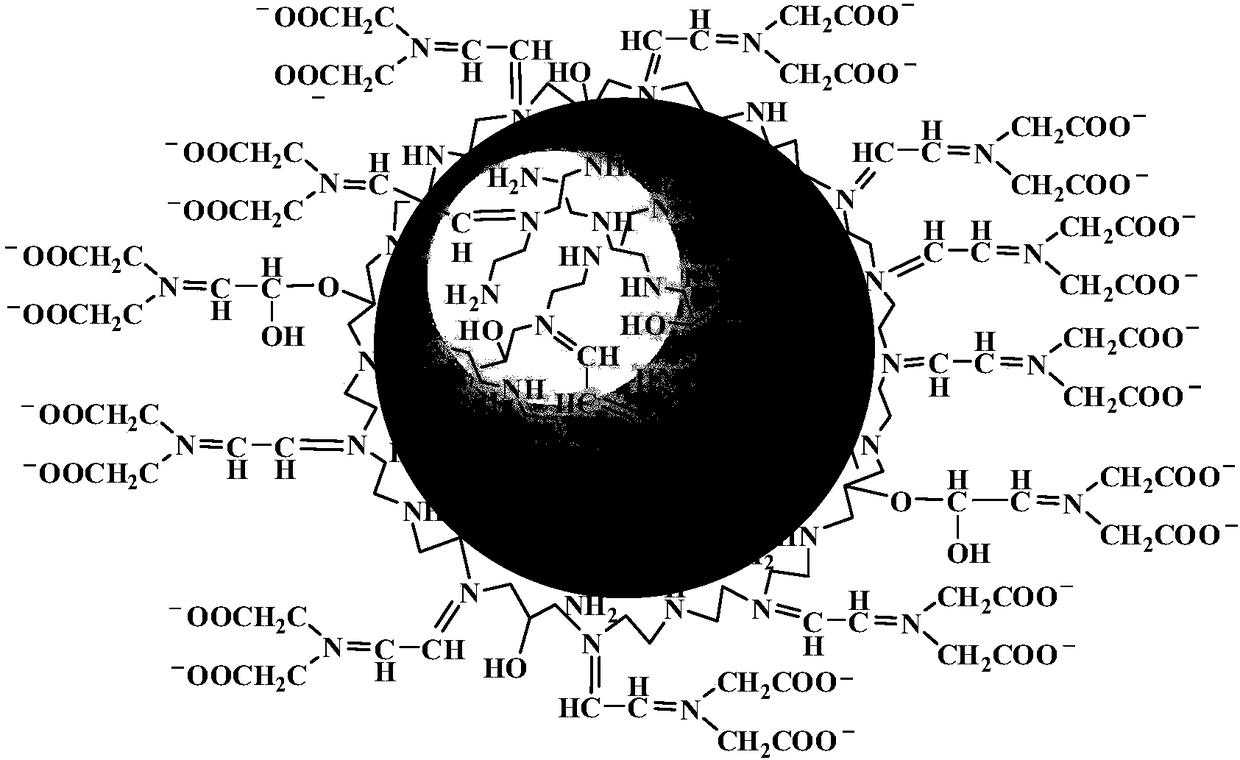
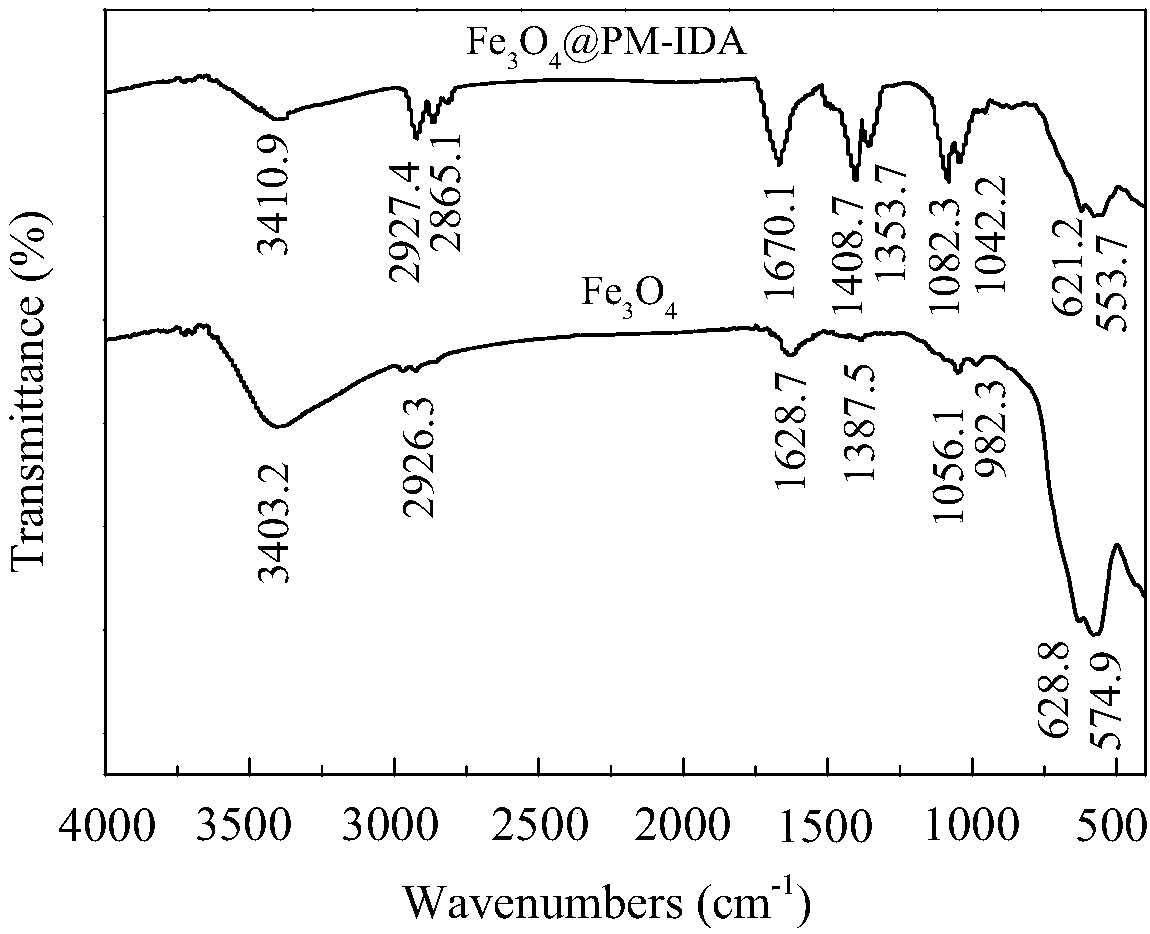
Solid magnetic heavy metal ion separation material and preparation method thereof
A technology for separating heavy metal ions and materials, applied in the fields of alkali metal compounds, chemical instruments and methods, alkali metal oxides/hydroxides, etc., can solve the problems of high manufacturing cost, small adsorption capacity, poor adaptability, etc., and achieve improved Uniformity and stability, the uniformity of the coating layer, and the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
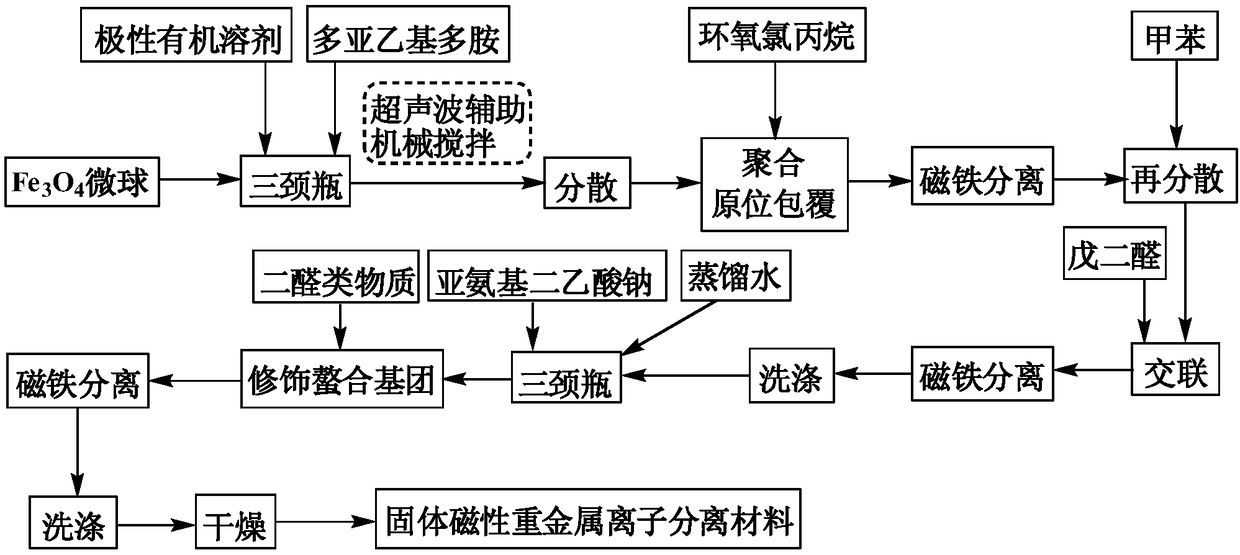
[0028] (1) Get 2.0g of ferric oxide microspheres with an average particle size of 435nm and 100mL of absolute ethanol into a 250mL three-necked flask with a dropping funnel, reflux condenser and mechanical stirring, then add 4.10g of quality Triethylenetetramine with a percentage concentration of 95.0%, mechanically stirred for 3 hours with the aid of ultrasonic waves. Raise the temperature to 60°C, and slowly add 2.4 mL of epichlorohydrin with a concentration of 99.0% by mass dropwise. After the dropwise addition, continue to react for another 3 h, cool to room temperature, and then adsorb and separate the solid matter with a magnet.
[0029] (2) Transfer the solid matter separated in step (1) into a 250mL three-necked flask with a dropping funnel, a reflux condenser and a mechanical stirrer, add 100mL toluene, and ultrasonically assisted mechanically stir for 3h, then pass through the dripping Slowly add a 5.0% chloroform solution prepared by 0.36mL glutaraldehyde into the f...
Embodiment 2
[0032] (1) Get 2.0g of ferric oxide microspheres with an average particle size of 340nm and 90mL of absolute ethanol and join them in a 250mL three-necked flask with a dropping funnel, reflux condenser and mechanical stirring, then add 4.50g of quality Triethylenetetramine with a percentage concentration of 95.0%, mechanically stirred for 2.5 hours with the aid of ultrasonic waves. Raise the temperature to 55°C, slowly add 2.6mL of epichlorohydrin with a mass percent concentration of 99.0% dropwise, and continue the reaction for 2.5h after the dropwise addition, cool to room temperature, and then adsorb and separate the solid matter with a magnet.
[0033] (2) transfer the solid matter separated in step (1) into a 250mL three-necked flask with a dropping funnel, a reflux condenser and a mechanical stirrer, add 90mL toluene, and ultrasonically assisted mechanically stir for 2h, then pass the dripping Slowly add 7.0% chloroform solution prepared by 0.33mL glutaraldehyde into the...
Embodiment 3
[0036] (1) Get 2.0g of ferric oxide microspheres with an average particle size of 218nm and 80mL of absolute ethanol into a 250mL three-necked flask with a dropping funnel, reflux condenser and mechanical stirring, then add 5.00g of quality Triethylenetetramine with a percentage concentration of 95.0%, mechanically stirred for 3 hours with the aid of ultrasonic waves. The temperature was raised to 50°C, and 2.97 mL of epichlorohydrin with a concentration of 99.0% by mass was slowly added dropwise. After the dropwise addition was completed, the reaction was continued for another 3 h, cooled to room temperature, and then the solid matter was adsorbed and separated by a magnet.
[0037] (2) Transfer the solid matter separated in step (1) into a 250mL three-necked flask with a dropping funnel, a reflux condenser and a mechanical stirrer, add 100mL toluene, and ultrasonically assisted mechanically stir for 3h, then pass through the dripping Slowly add a 10.0% chloroform solution pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com