Atherosclerotic plaque-targeting platelet membrane coated rapamycin bionic nano particles and application thereof
A technology of atherosclerosis and rapamycin, applied in the field of medicine, can solve the problems of endangering unstable plaques and counteracting anti-plaque efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
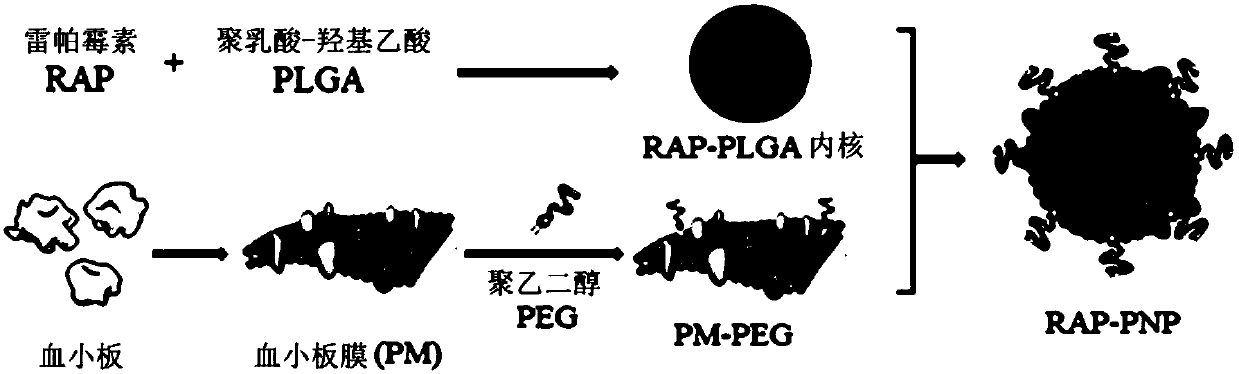
[0042] Example 1: Synthesis of biomimetic nanoparticles of rapamycin coated with platelet membrane
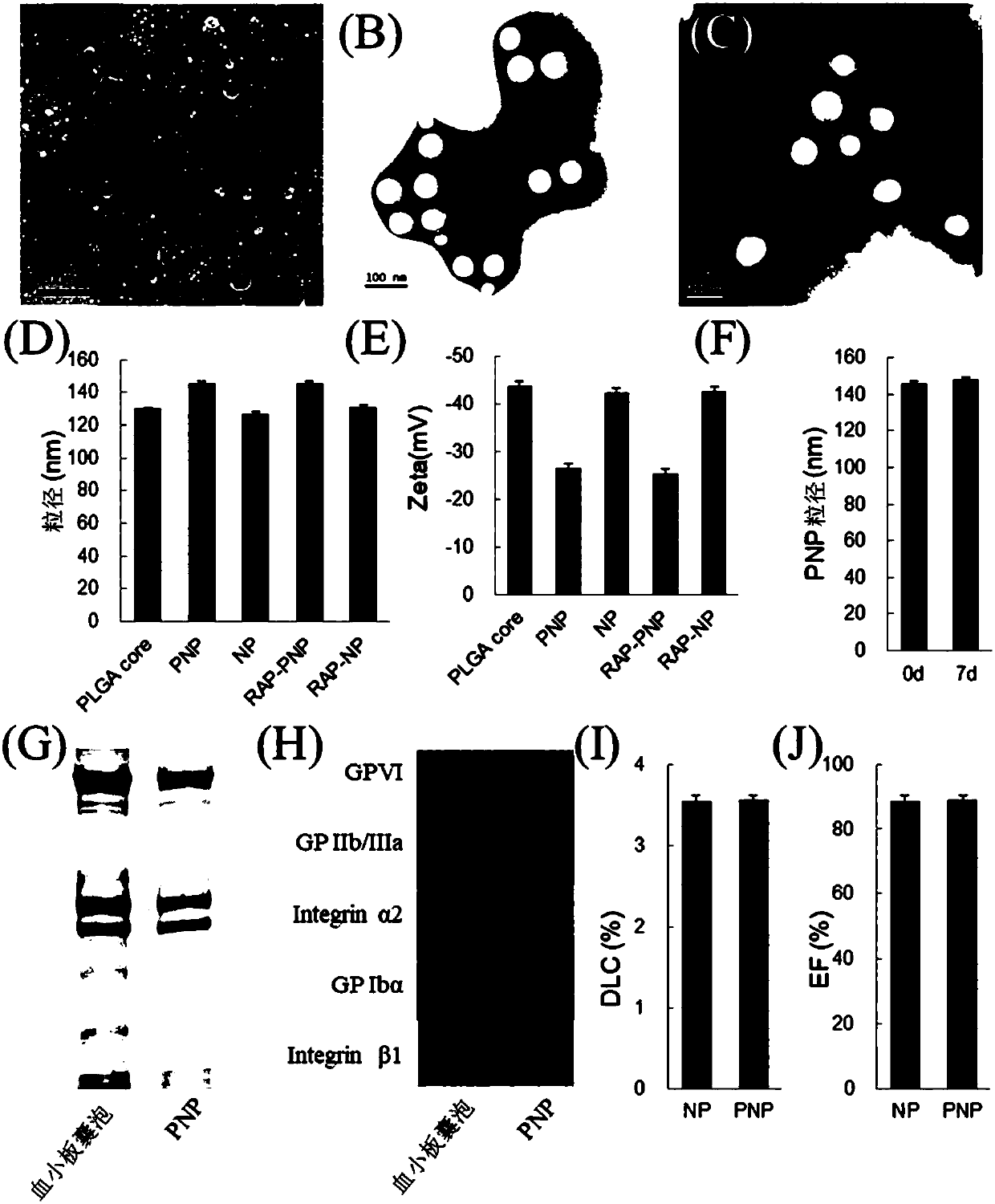
[0043] Synthesis schematic see figure 1 . 800 μg of rapamycin and 20 mg of PLGA were dissolved in 1 ml of acetone, injected into 2 ml of water, and PLGA nanoparticles loaded with rapamycin were prepared by nanoparticle precipitation method under vacuum. After the human platelet-rich plasma was anticoagulated with EDTA, it was centrifuged at 100g for 20min to remove the remaining blood cells. Add EDTA and prostacyclin to the supernatant to inhibit platelet activation, centrifuge at 800 g for 20 min, discard the supernatant, and resuspend the platelets in PBS containing EDTA and protease inhibitors. 3×10 9 Platelets were used to coat 1 mg PLGA nanoparticles. Platelets Platelet membranes were prepared using the repeated freeze-thaw method. After the platelets were quick-frozen with liquid nitrogen, they were dissolved at room temperature and repeated three times, centrifuged ...
Embodiment 2
[0045] Example 2: PNP simulates platelet adhesion function in vitro
[0046] The adhesion function of platelets was simulated by combining PNP with collagen, fibrin, vWF, etc. under static and dynamic conditions in vitro.
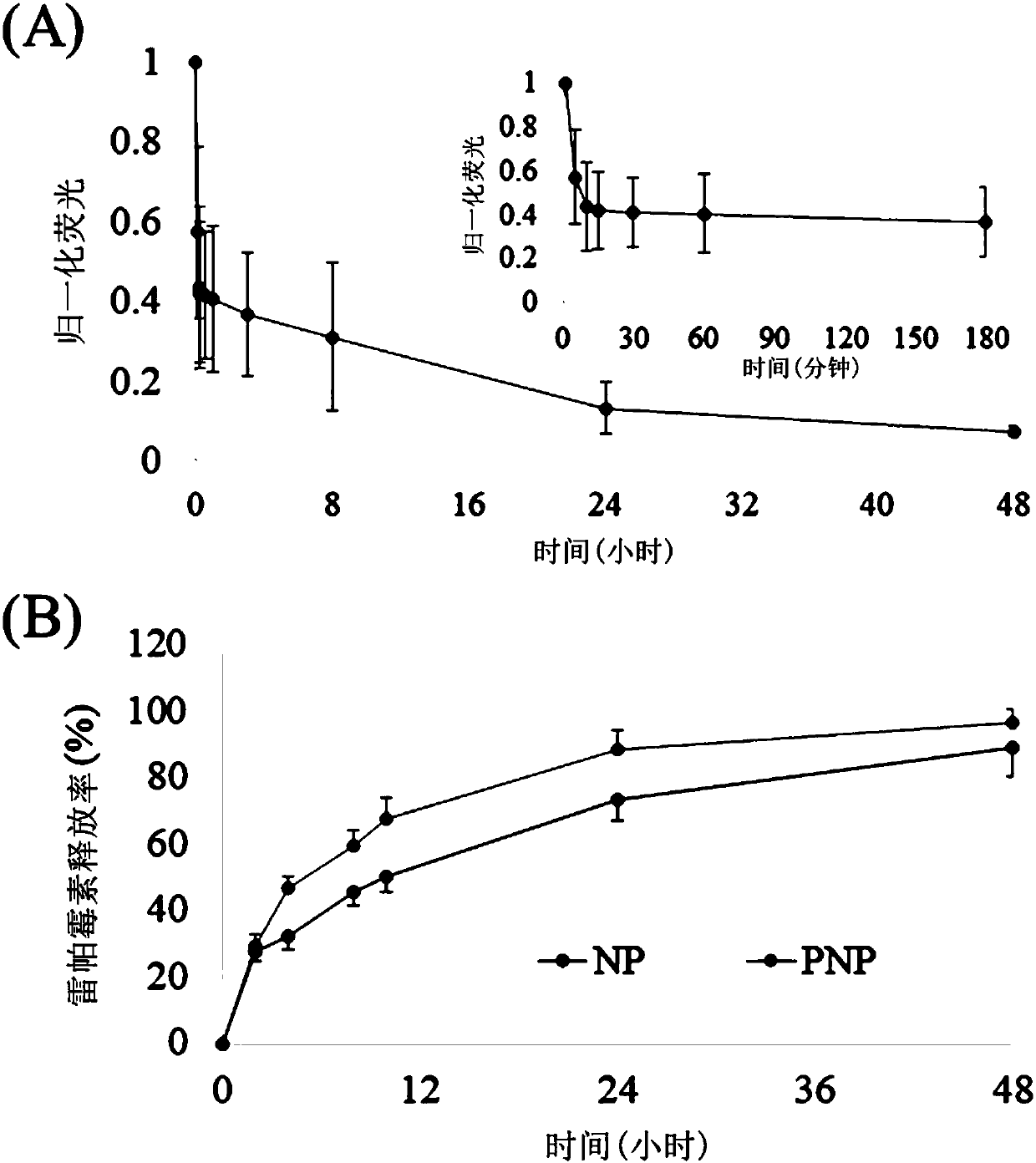
[0047] Binding of PNP to collagen and fibrin in a static state: After adding collagen or fibrin to a 96-well plate, add DiD-labeled PNP, incubate at 37°C for 5 minutes, wash with PBS, dissolve the bound PNP with DMSO, and measure with a microplate reader its fluorescence intensity. As shown in 4A and B, PNP has a strong binding force to collagen and fibrin, which are 8.3 times and 9.6 times that of uncoated platelet membrane nanoparticles (NP), respectively, and there is a significant difference between the two.
[0048] The combination of PNP with collagen, fibrin and vWF in a dynamic state: use glass slides to prepare collagen and fibrin, place them in a parallel plate flow chamber, DiD-labeled PNP flows through the parallel plate flow chamber at 500S-1,...
Embodiment 3
[0050] Example 3: PNP binds to atherosclerotic plaque in vitro
[0051] In Vitro Binding Ability of PNP to Mouse Atherosclerotic Plaques: ApoE - / - After mice were fed with a high-fat diet for 8 weeks, the aorta was removed and co-incubated with DiD-labeled PNP for imaging of isolated organs, such as Figure 4 F and G, the fluorescence intensity of the PNP group was significantly higher than that of the control group NP, and it was mainly distributed in the area rich in plaques (the branch of the brachiocephalic trunk and the subclavian artery). strong binding.
[0052] The in vitro binding ability of PNP and human carotid atherosclerotic plaque: After the human carotid plaque was taken out, it was co-incubated with DiD-labeled PNP, and after OCT embedding, it was frozen and sectioned, and laser confocal microscope observation showed that the PNP group had more Red fluorescence (PNP) adhered, while no obvious red fluorescence was seen in the NP group (such as Figure 4 H sho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com