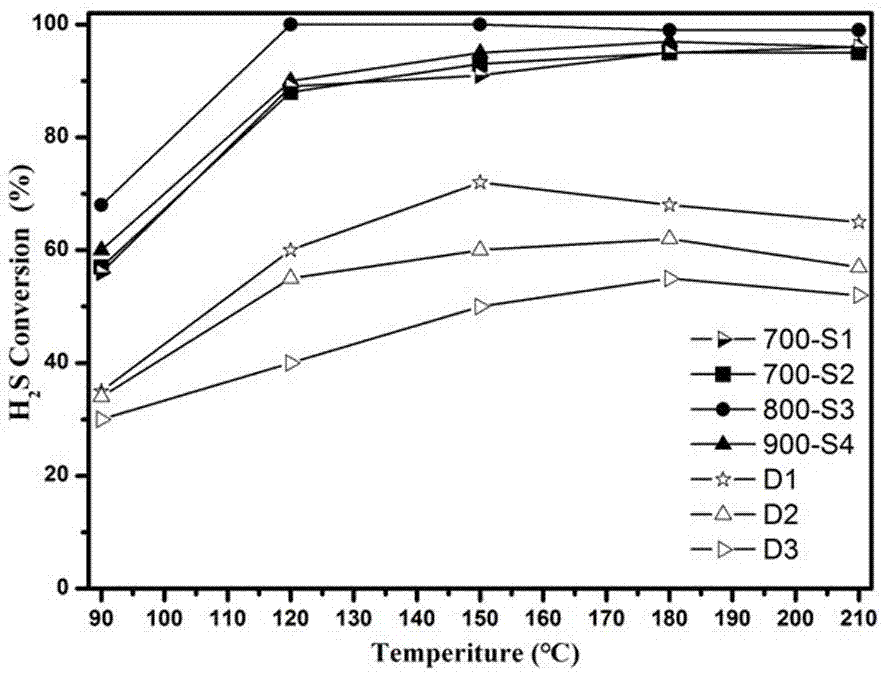
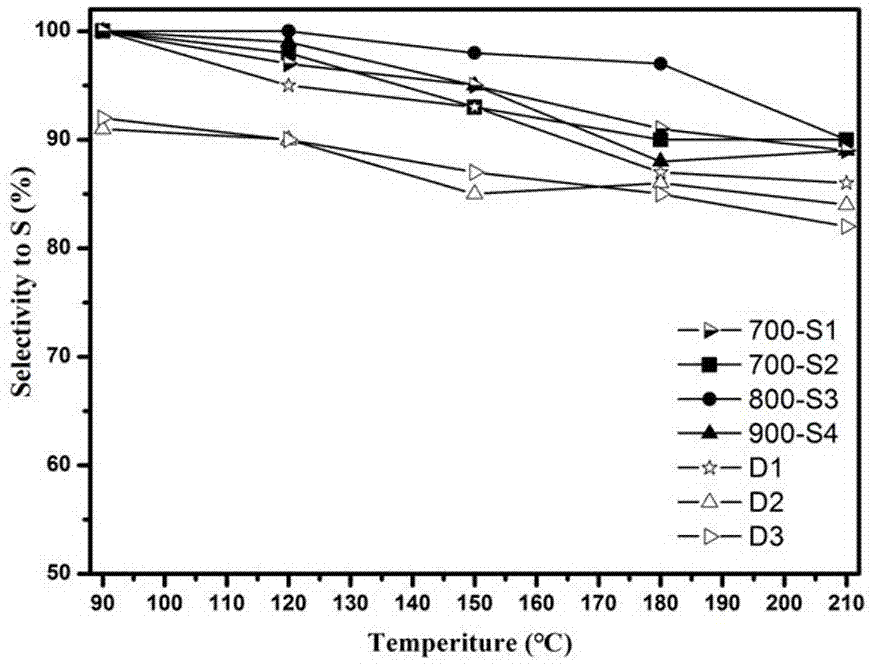
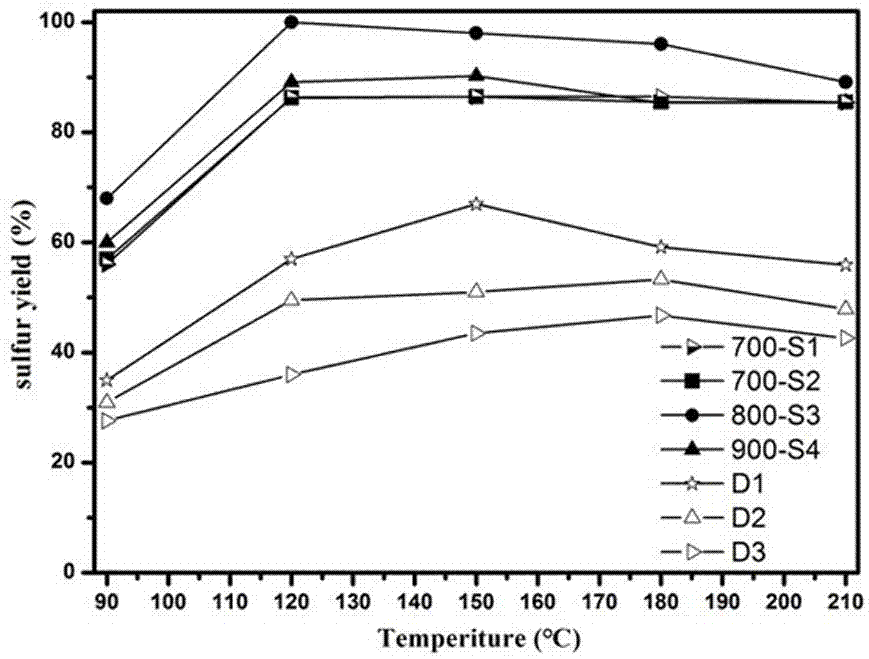
Preparation method of catalyst for H2S selective catalytic oxidation
A catalytic oxidation and catalyst technology, which is applied in the preparation/purification of sulfur, chemical instruments and methods, separation methods, etc., can solve the problems of cumbersome preparation process, easy loss of active components, and few catalytic centers, and achieves a simple and easy synthesis method. , the effect of excellent catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]Weigh 0.654g 3-aminophenol, 0.42g HMTA (hexamethylenetetramine), dissolve it in 75ml deionized water, and wait until completely dissolved to form a clear solution A; weigh 0.47g F127 template and dissolve it in 5ml deionized water , to be completely dissolved to form a clear solution B; then slowly add solution A to solution B during stirring to form a mixed solution C, and then stir the mixed solution C in an oil bath at 50°C for 16 hours, and after 16 hours, the mixture Transfer to a clean polytetrafluoroethylene-lined kettle and place it in an oven at 95°C for 12 hours. When it cools down to room temperature naturally, take out the reaction kettle, filter, wash, and dry to collect samples. The samples collected in the previous step were placed in a porcelain ark and placed in a tube furnace for pre-oxidation in an air atmosphere at 250 °C for 2 h with a heating rate of 1 °C / min. Put the pre-oxidized sample into a tube furnace with a nitrogen atmosphere, first roast it...
Embodiment 2
[0029] Weigh 3.27g of 3-aminophenol, 2.2g of HMTA (hexamethylenetetramine), dissolve in 375ml of deionized water, until completely dissolved to form a clear solution A; weigh 2.35g of F127 template and dissolve in 25ml of deionized water , to be completely dissolved to form a clear solution B; then slowly add solution A to solution B during stirring to form a mixed solution C, and then stir the mixed solution C in an oil bath at 50°C for 16 hours, and after 16 hours, the mixture Transfer to a clean polytetrafluoroethylene-lined kettle, and keep it in an oven at 95°C for 12 hours. When it is naturally cooled to room temperature, take out the reaction kettle, filter, wash, and dry to collect samples. The samples collected in the previous step were placed in a porcelain ark and placed in a tube furnace for pre-oxidation in an air atmosphere at 250°C for 2 hours with a heating rate of 1°C / min. Put the pre-oxidized sample into a tube furnace with a nitrogen atmosphere, first roast ...
Embodiment 3
[0030] Embodiment 3 (best embodiment):
[0031] Weigh 0.654g 3-aminophenol, 0.42g HMTA (hexamethylenetetramine), dissolve it in 75ml deionized water, and wait until completely dissolved to form a clear solution A; weigh 0.47g F127 template and dissolve it in 5ml deionized water , to be completely dissolved to form a clear solution B; then slowly add solution A to solution B during stirring to form a mixed solution C, and then stir the mixed solution C in an oil bath at 50°C for 16 hours, and after 16 hours, the mixture Transfer to a clean polytetrafluoroethylene-lined kettle, and react in a constant temperature oven at 95°C for 12 hours. When it is naturally cooled to room temperature, take out the reactor, filter, wash, and dry to collect samples. The samples collected in the previous step were placed in a porcelain ark and placed in a tube furnace for pre-oxidation in an air atmosphere at 250 °C for 2 h with a heating rate of 1 °C / min. Put the pre-oxidized sample into a tub...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com