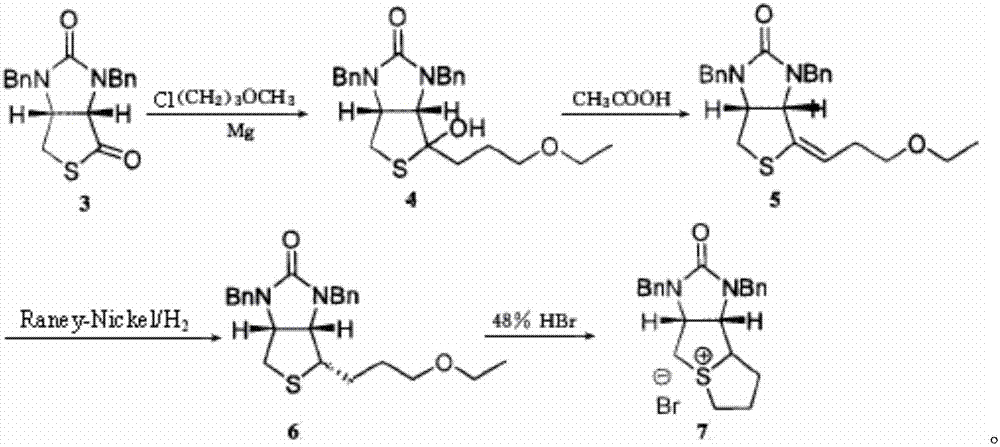
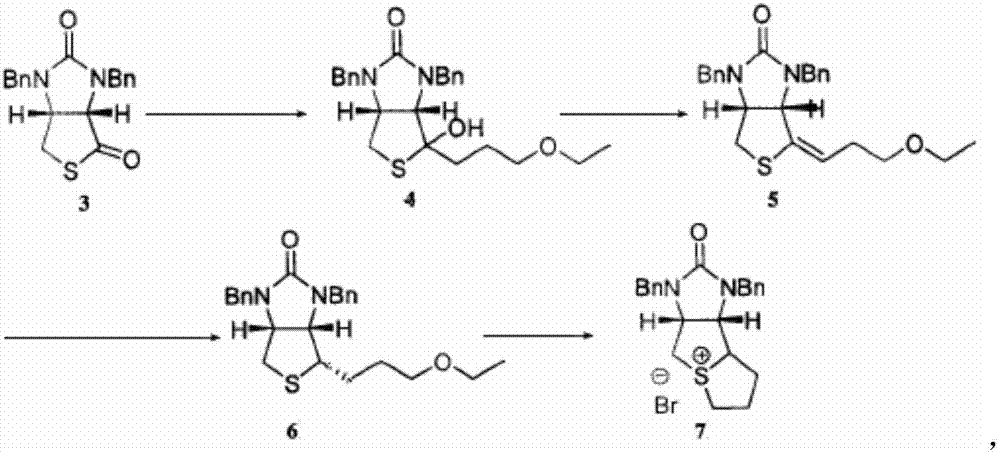
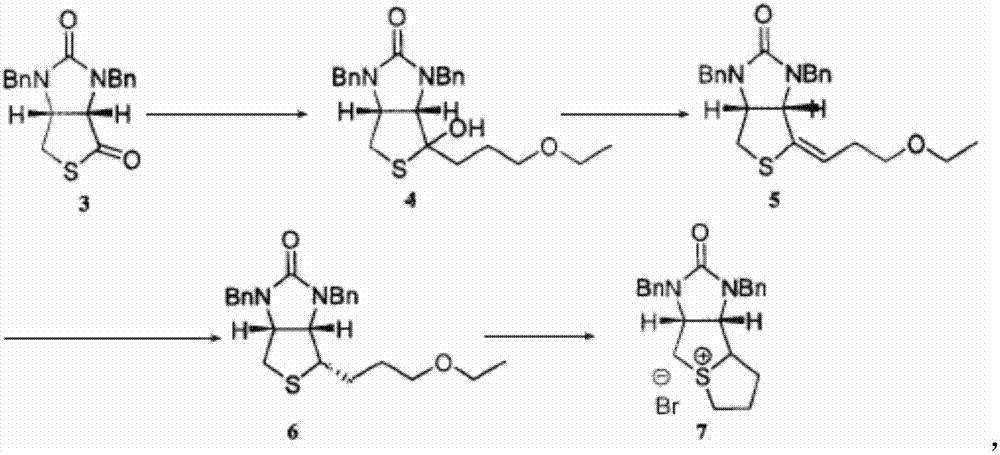
Method for preparing D-biotin bromide intermediate
An intermediate, biotin technology, which is applied in the field of preparing D-biotin bromide salt intermediate, can solve the problems of violent initiation reaction, high production cost, large dosage, etc., achieve simple and safe operation, mild reaction conditions, and realize recovery and regeneration The effect of using
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Preparation of 1-bromo-3-methoxypropane Grignard reagent
[0035] Reference: The method described in the Chemical Journal of Chinese Universities, 2001, 27(7), 1141-1146 prepares the initially initiated 1-bromo-3-methoxypropane magnesium bromide Grignard reagent.
[0036] Put 40kg of dried magnesium chips and 1000L of tetrahydrofuran into a dry and anhydrous reaction kettle, stir and raise the temperature to 30-40°C, add 3L of 1-bromo-3-methoxypropane magnesium bromide Grignard reagent, 1-bromo- 10L of 3-methoxypropane; after the reaction is initiated, the temperature will rise automatically, control the temperature at 40-60°C, and then add 200L of 1-bromo-3-methoxypropane dropwise; to 20°C; transfer the obtained Grignard reagent to another reaction vessel for use, while keeping a small amount of Grignard reagent in the still as an initiator for the next preparation of the Grignard reagent.
Embodiment 2
[0037] Embodiment 2: prepare bromine salt intermediate
[0038] S1) Put 1500L of toluene and 230kg of compound 3 into the dissolution tank, stir to dissolve the solid, and obtain the toluene solution of compound 3; slowly drop the obtained toluene solution of compound 3 into 1-bromo-3-methoxypropane bromide In the magnesium oxide Grignard reagent, the temperature in the reaction system is controlled to be 50-60°C during the dropping process; after the dropping is completed, keep warm and stir until the reaction is completed; concentrate under reduced pressure until no liquid flows out, add 400L toluene to the residue and stir to dissolve, Then add 300L of 12% sulfuric acid aqueous solution, stir for 1 hour; leave to stand for stratification, wash the collected organic layer with 400L of water twice, and then wash once with 100L of saturated brine to obtain a toluene solution of compound 4;
[0039]S2) Add 1kg of p-toluenesulfonic acid to the toluene solution of compound 4, hea...
Embodiment 3
[0042] Embodiment 3: to the regeneration of the palladium charcoal catalyst that reclaims
[0043] Put 40kg of waste palladium carbon into the hydrogenation kettle, add 600L of ethanol, fully replace with nitrogen, raise the temperature to 150-160°C, then slowly introduce hydrogen to 5MPa, and then keep warm and pressurize for 4-5 hours; filter, use ethanol and water The filter cake is fully washed successively, and the catalyst regeneration is completed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com