Synthetic method of benzenesulfonate derivative
A technology of benzenesulfonate ester and synthesis method, which is applied in the field of synthesis of benzenesulfonate derivatives, can solve the problems of complex synthesis method, battery performance degradation, damage, etc., and achieve simple synthesis method and enhanced high and low temperature stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
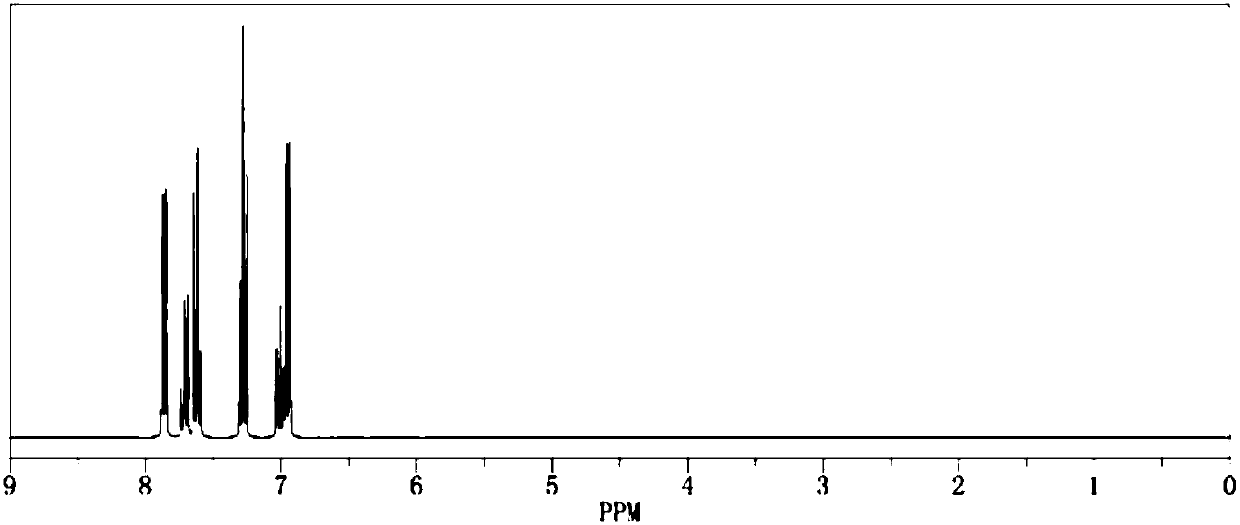
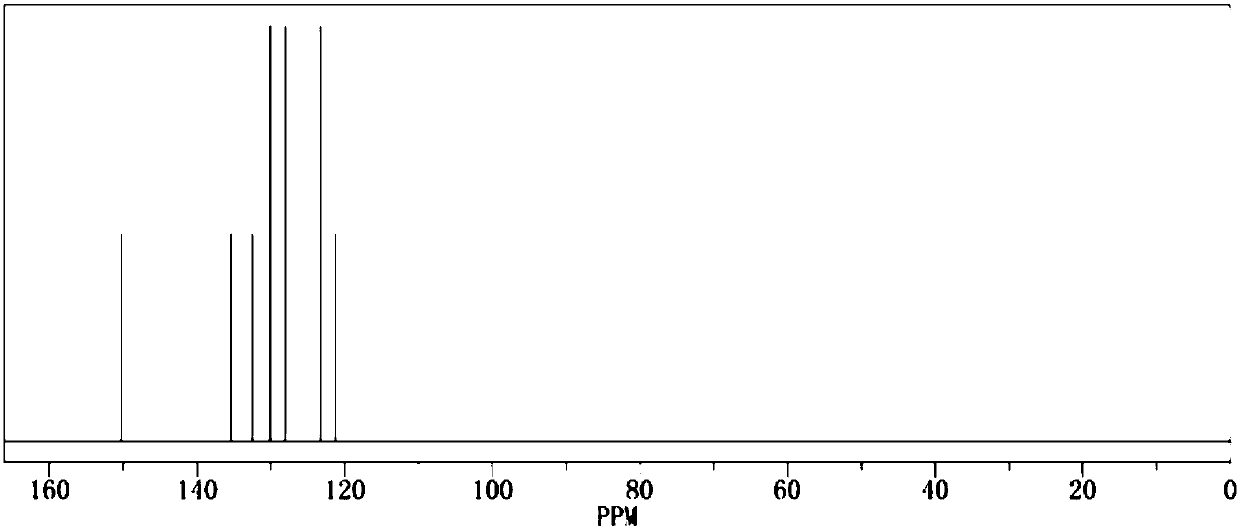
[0020] Add 1.0mol of phenol and 500ml of dichloromethane into the reaction flask respectively, add triethylamine under stirring, then cool down to below 15°C, start to add 1.1mol of benzenesulfonyl chloride dropwise, return to room temperature and continue stirring for 1h, then raise the temperature The reaction was continued at reflux for 2 hours. After the reaction was completed by gas phase detection, it was ice-thawed, separated, dried and concentrated to obtain the crude product and then recrystallized to obtain 222.3 g of the pure product. The calculated yield of the product was 95%. The detection purity is 99.93%, the moisture content is 30ppm, the acid value is 34ppm, and the measured density is 1.277g / cm 3 , with a boiling point of 375.4°C and 760mmHg, and its 1H NMR spectrum is as follows figure 1 As shown, the 13CNMR spectrum is as figure 2 shown.
[0021] The synthetic route is:
[0022]
Embodiment 2
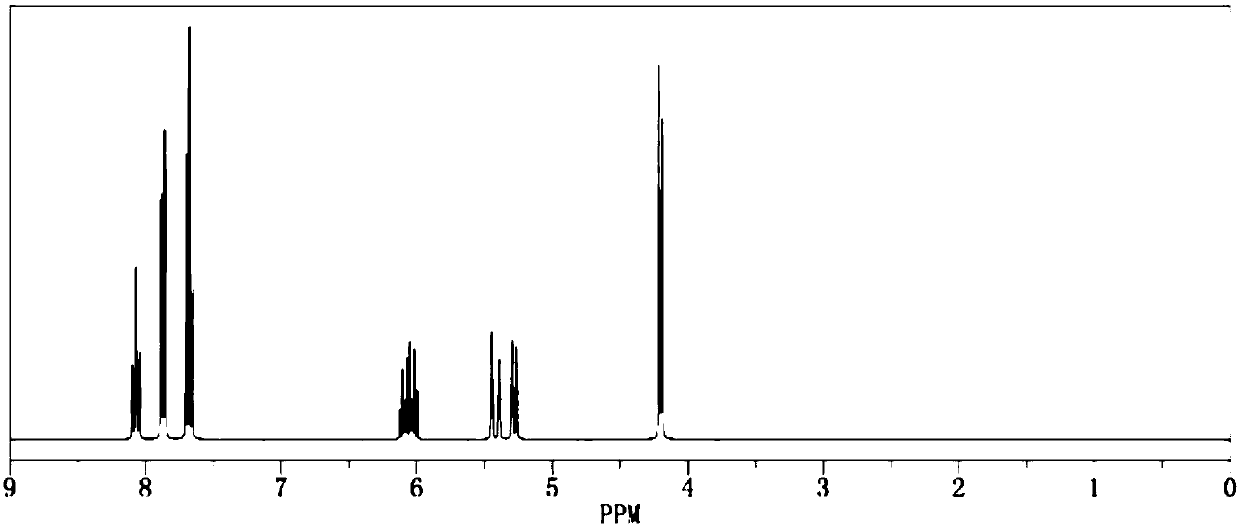
[0024] Add 1.0mol of allyl alcohol and 500ml of dichloromethane into the reaction flask respectively, add pyridine under stirring, then cool down to below 15°C, start to add 2.1mol of benzenesulfonyl chloride dropwise, return to room temperature and continue stirring for 1h, and then raise the temperature to The reaction was continued under reflux for 2 hours. After the reaction was completed by gas phase detection, it was ice-thawed, separated into layers, dried and concentrated to obtain the crude product and then recrystallized to obtain 189.3 g of the pure product. The calculated yield of the product was 95.5%. The detection purity is 99.95%, the moisture content is 30ppm, and the acid value is 40ppm, and its 1H NMR spectrum is as image 3 As shown, the 13C NMR spectrum is as Figure 4 shown.
[0025] The synthetic route is:
[0026]
Embodiment 3
[0028] Add 1.0mol of propargyl alcohol and 500ml of dichloromethane into the reaction flask respectively, add triethylamine under stirring, then lower the temperature to below 15°C, start to add 2.1mol of benzenesulfonyl chloride dropwise, return to room temperature and continue stirring for 1h, then The temperature was raised to reflux to continue the reaction for 2 hours. After the reaction was completed by gas phase detection, it was ice-thawed, separated, dried and concentrated to obtain the crude product and then recrystallized to obtain 187.77 g of the pure product. The calculated yield was 95.8%. The detection purity is 99.93%, the moisture content is 28ppm, the acid value is 36ppm, and the density is 1.244g / mL.
[0029] The synthetic route is:
[0030]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Boiling point | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com