Theophylline slow-release tablet and preparation method thereof
A technology of slow-release tablets and theophylline, which is applied in the field of medicine, can solve problems such as poor release linearity, large difference in release degree of theophylline sustained-release tablets, and unfavorable therapeutic effect of theophylline, so as to solve diseases, improve use value, and avoid Toxic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
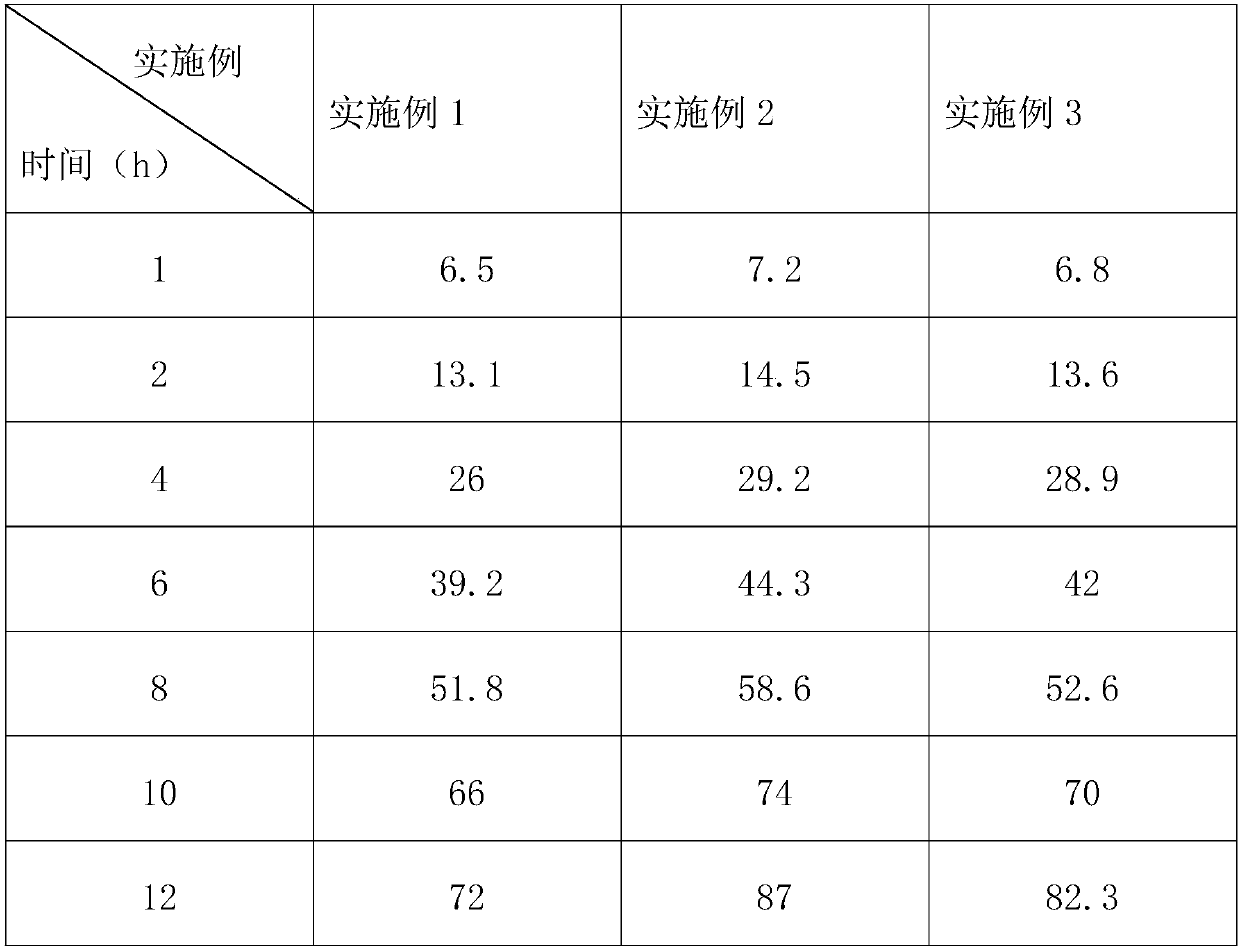
Embodiment 1
[0028] A theophylline sustained-release tablet and a preparation method thereof, consisting of a tablet core and a coating layer, calculated in parts by weight, the tablet core includes the following raw and auxiliary materials: 60 parts of theophylline, 5 parts of bellflower, 0.5 part of tangerine peel, 8 parts of licorice
[0029] 0.3 parts of almonds, 3 parts of butter; the coating layer includes the following raw materials: 10 parts of titanium dioxide, 15 parts of polyvinylpyrrolidone, 0.3 parts of beeswax, 0.6 parts of carnauba wax, and 0.6 parts of sucrose.
[0030] Among them, platycodon grandiflorum is soaked in 90% ethanol solution for 12-24 hours, rinsed with distilled water for 30 minutes, dried until the water content is lower than 8%, and crushed to a size of 500-800 mesh under the action of supersonic airflow.
[0031] A preparation method for theophylline sustained-release tablets, comprising the steps of:
[0032] (1) Superfinely pulverize tangerine peel, lico...
Embodiment 2
[0039] A theophylline sustained-release tablet and a preparation method thereof, comprising a tablet core and a coating layer, calculated in parts by weight, the tablet core includes the following raw and auxiliary materials: 80 parts of theophylline, 8 parts of bellflower, 0.8 part of tangerine peel, and 12 parts of licorice , 0.6 parts of almonds, 6 parts of lard; the coating layer includes the following raw materials: 12 parts of titanium dioxide, 35 parts of ethyl acrylate-methyl methacrylate copolymer, 0.8 parts of beeswax, 0.9 parts of carnauba wax, and 0.8 parts of sucrose.
[0040] Among them, platycodon grandiflorum is soaked in 90% ethanol solution for 12-24 hours, rinsed with distilled water for 30 minutes, dried until the water content is lower than 8%, and crushed to a size of 500-800 mesh under the action of supersonic airflow.
[0041] A preparation method for theophylline sustained-release tablets, comprising the steps of:
[0042] (1) Superfinely pulverize tan...
Embodiment 3
[0049]A theophylline sustained-release tablet and a preparation method thereof, consisting of a tablet core and a coating layer, calculated in parts by weight, the tablet core includes the following raw and auxiliary materials: 70 parts of theophylline, 6 parts of bellflower, 0.6 part of tangerine peel, 10 parts of licorice , 0.38 parts of almonds, 3 parts of mutton oil; the coating layer includes the following raw materials: 10-12 parts of titanium dioxide, 15 parts of methyl cellulose, 0.3 parts of beeswax, 0.6 parts of carnauba wax, and 0.6 parts of sucrose.
[0050] Among them, platycodon grandiflorum is soaked in 90% ethanol solution for 12-24 hours, rinsed with distilled water for 30 minutes, dried until the water content is lower than 8%, and crushed to a size of 500-800 mesh under the action of supersonic airflow.
[0051] A preparation method for theophylline sustained-release tablets, comprising the steps of:
[0052] (1) Superfinely pulverize tangerine peel, licoric...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com