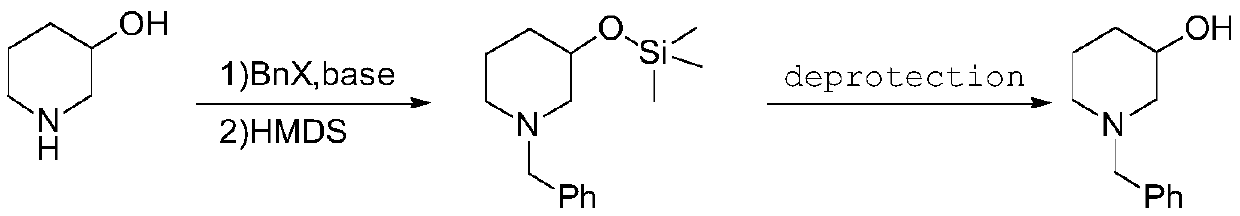
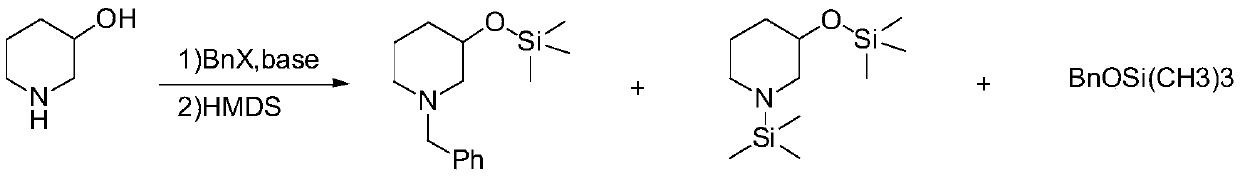A kind of synthetic method of n-benzyl-3-hydroxypiperidine
A technology of hydroxypiperidine and a synthesis method, which is applied in the synthesis field of N-benzyl-3-hydroxypiperidine, can solve the problems of poor conversion rate and selectivity, incomplete conversion of raw materials, easy occurrence of accidents and the like, and achieves the Avoid the purification process of vacuum distillation, the effect of good reaction conversion and selectivity, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] In the first step, in a 2L three-necked flask equipped with magnetic stirring, 3-hydroxypiperidine (101.2g, 1mol) was dissolved in 1L toluene, potassium carbonate (276.4g, 2mol) was added, and 0.9 equivalents of benzyl bromide was added dropwise (153.9g, 0.9mol), reacted at 40-50°C for 5 hours, and the reaction was controlled by GC; Hours, TLC detects that the reaction is complete, and the developing agent is: n-hexane / ethyl acetate=5: 1. After cooling down and concentrating the solvent, the low-boiling substance (42~69°C, 2torr) is distilled off under reduced pressure, and the residue is distilled at room temperature. Add 1.2L methyl tert-butyl ether to the solution, filter out the insoluble matter, the filtrate is the methyl tert-butyl ether solution of N-benzyl-3-trimethylsilyloxypiperidine, which is directly used in the next step reaction, GC Purity: 97.6% (excluding solvent), GCMS (m / z): 263.17.
[0027] In the second step, in a 2L three-neck flask equipped with m...
Embodiment 2
[0029] In the first step, in a 2L three-necked flask equipped with magnetic stirring, 3-hydroxypiperidine (101.2g, 1mol) was dissolved in 1L of acetone, cesium carbonate (448.7g, 1.5mol) was added, and 0.95 equivalents of chloride Benzyl (120.3g, 0.95mol) was reacted at 40-50°C for 3 hours, and the reaction was controlled by GC; under the protection of nitrogen, hexamethyldisilazane (242.1g, 1.5mol) was added, and the temperature was raised to reflux for 2 hours. TLC detects that the reaction is complete. The developer is: n-hexane / ethyl acetate=5:1. After cooling down and concentrating the solvent, the low boiling point substance (42~69°C, 2torr) is distilled off under reduced pressure, and added to the residue at room temperature. 1.2L methyl tert-butyl ether, filter out the insoluble matter, the filtrate is the methyl tert-butyl ether solution of N-benzyl-3-trimethylsiloxypiperidine, which is directly used in the next step reaction, GC purity: 97.7% (deducting solvent).
...
Embodiment 3
[0032] In the first step, in a 2L three-necked flask equipped with magnetic stirring, dissolve 3-hydroxypiperidine (101.2g, 1mol) in 1L tetrahydrofuran, add sodium carbonate (265.0g, 2.5mol), and dropwise add 0.95 equivalents of brominated Benzyl (162.5g, 0.95mol) was heated and refluxed for 4 hours, and the reaction was controlled by GC; under the protection of nitrogen, hexamethyldisilazane (322.8g, 2mol) was added, heated and refluxed for 2 hours, and the reaction was detected by TLC After completion, the developing solvent is: n-hexane / ethyl acetate=5:1, lower the temperature, concentrate the solvent, distill off the low boiling point substance (42~69°C, 2torr) under reduced pressure, add 1.2L formazan to the residue at room temperature Methyl tert-butyl ether, filter out insolubles, the filtrate is the methyl tert-butyl ether solution of N-benzyl-3-trimethylsiloxy piperidine, directly used in the next step reaction, GC purity: 97.6% ( minus the solvent).
[0033] In the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com